Culture medium of meningitis Neisseria
A technology of Neisseria and culture medium, applied in the direction of bacteria, microorganism-based methods, microorganisms, etc., can solve the problems of unsuitable for large-scale enrichment, difficult source of materials, complicated production process, etc., and achieve a beneficial effect on colonies The effects of expansion, process simplification, and wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prepare according to the following formula:
[0020] Sodium chloride 5.8g Potassium chloride 0.09g
[0021] Disodium hydrogen phosphate 2.6g L-glutamic acid 15.0g
[0022] L-Arginine 0.03g L-Tryptophan 0.0025g
[0023] L-cysteine 0.012g Calcium chloride 0.021g
[0024] Ferrous sulfate 0.002g Magnesium sulfate 0.6g
[0025] Soy peptone 15.0g Glucose 10.0g
[0026] Prepared by the following method:
[0027] A. Dissolve the mixture of sodium chloride, potassium chloride, disodium hydrogen phosphate, L-glutamic acid, L-arginine, L-cysteine, calcium chloride, ferrous sulfate and soybean peptone in distilled water , adjust the volume to 1L, adjust the pH to 7.4, and sterilize at 113°C and 0.08MPa for 15min;
[0028] B. Dissolve the mixture of L-tryptophan, magnesium sulfate and glucose in 15 mL of distilled water, filter and sterilize with a 0.2 μm disposable filter, and then add it to the mixture in step A that has been autoclaved to obtain a liquid medium.
Embodiment 2
[0030] Prepare according to the following formula:
[0031] Sodium chloride 3.0g Potassium chloride 0.05g
[0032] Disodium hydrogen phosphate 6.0g L-glutamic acid 5.0g
[0033] L-Arginine 0.01g L-Tryptophan 0.001g
[0034] L-cysteine 0.005g Calcium chloride 0.01g
[0035] Ferrous sulfate 0.008g Magnesium sulfate 1.0g
[0036] Soy peptone 5.0g Glucose 5.0g
[0037] Prepared by the following method:
[0038] A. Dissolve the mixture of sodium chloride, potassium chloride, disodium hydrogen phosphate, L-glutamic acid, L-arginine, L-cysteine, calcium chloride, ferrous sulfate and soybean peptone in distilled water , adjust the volume to 1L, adjust the pH to 7.2, and sterilize at 117°C and 0.06MPa for 15 minutes;
[0039] B. Dissolve the mixture of L-tryptophan, magnesium sulfate and glucose in 15 mL of distilled water, filter and sterilize with a 0.2 μm disposable filter, and then add it to the mixture in step A that has been autoclaved to obtain a liquid medium.
Embodiment 3
[0041] Prepare according to the following formula:
[0042] Sodium chloride 8.0g Potassium chloride 0.2g
[0043] Disodium hydrogen phosphate 2.0g L-glutamic acid 20.0g
[0044] L-Arginine 0.1g L-Tryptophan 0.01g
[0045] L-cysteine 0.03g Calcium chloride 0.1g
[0046] Ferrous sulfate 0.001g Magnesium sulfate 0.1g
[0047] Soy peptone 20.0g Glucose 20.0g
[0048] Prepared by the following method:
[0049] A. Dissolve the mixture of sodium chloride, potassium chloride, disodium hydrogen phosphate, L-glutamic acid, L-arginine, L-cysteine, calcium chloride, ferrous sulfate and soybean peptone in distilled water , adjust the volume to 1L, adjust the pH to 7.4, and sterilize at 115°C and 0.07MPa for 20min;
[0050] B. Dissolve the mixture of L-tryptophan, magnesium sulfate and glucose in 15 mL of distilled water, filter and sterilize with a 0.2 μm disposable filter, and then add it to the mixture in step A that has been autoclaved to obtain a liquid medium.
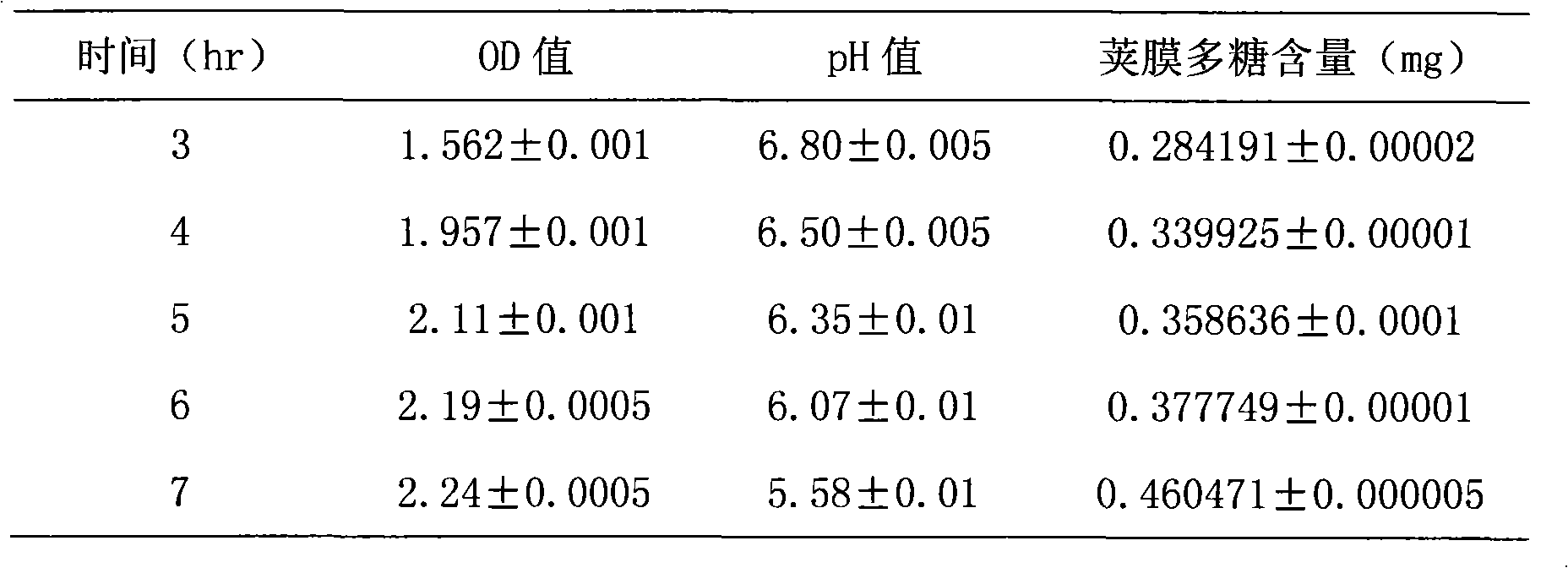
[0051] Neisse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com