Biological polypeptide medical device and manufacturing method thereof
A bio-peptide, medical device technology, applied in the direction of surface coating devices, stents, anodizing, etc., can solve the problems of unsafe, biological products falling off, falling off, etc., to avoid inflammation and side effects, to ensure effectiveness, The effect of specific capture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 penicillamine cyclic peptide scaffold
[0059] The 316L stainless steel vascular stent was cut, slag-removed, and polished, placed in 75% medical alcohol, cleaned with ultrasonic waves at a frequency of 100khz for 10 minutes, set the temperature at 40°C, dried for 40 minutes, and then taken out.
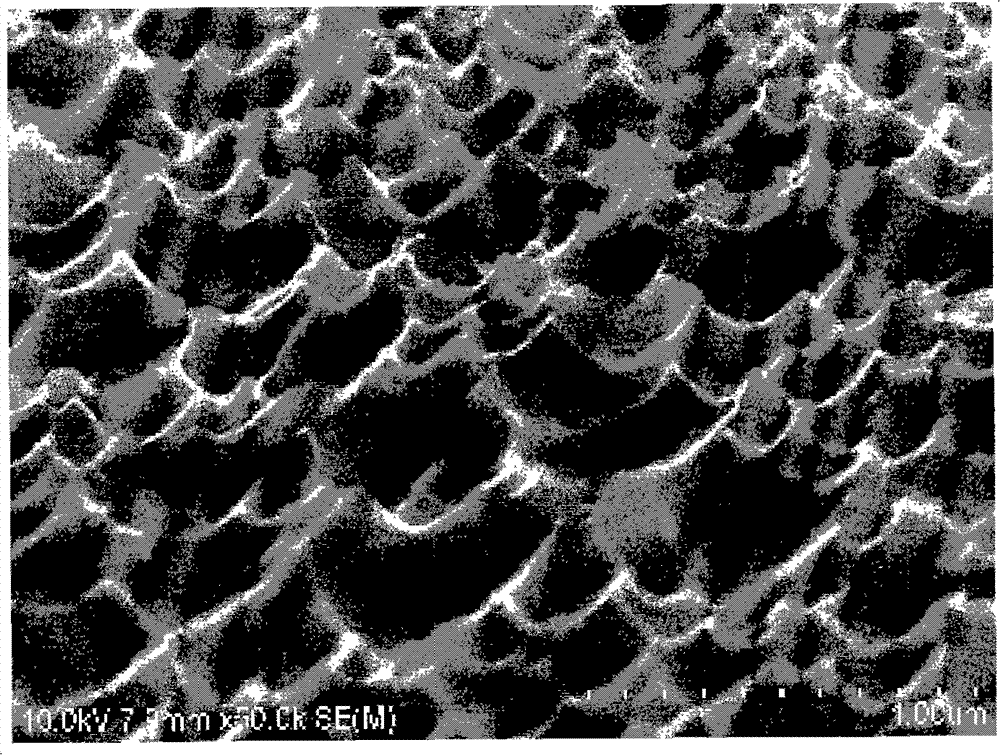
[0060] Then the support is placed in 15% hydrochloric acid corrosion for 12 hours, the support body is connected to the positive pole of the pulse power supply as the anode, and the titanium metal sheet is connected to the negative pole of the pulse power supply as the cathode, and the support body and the cathode metal sheet are simultaneously placed in a concentration of 15% hydrochloric acid, the current is set to 1A, the frequency is 500 Hz, and the time is 15 minutes, holes are prepared on the surface of the 316L bare metal stent body. Such as figure 1 As shown, under the electron microscope scanning, it can be seen that there are obvious ...
Embodiment 2c
[0063] Example 2 Preparation of cyclo-RGD-SAA polypeptide scaffold
[0064] The 316L stainless steel vascular stent was cut, slag-removed, and polished, placed in 75% medical alcohol, cleaned with ultrasonic waves at a frequency of 100khz for 10 minutes, set the temperature at 40°C, dried for 40 minutes, and then taken out.
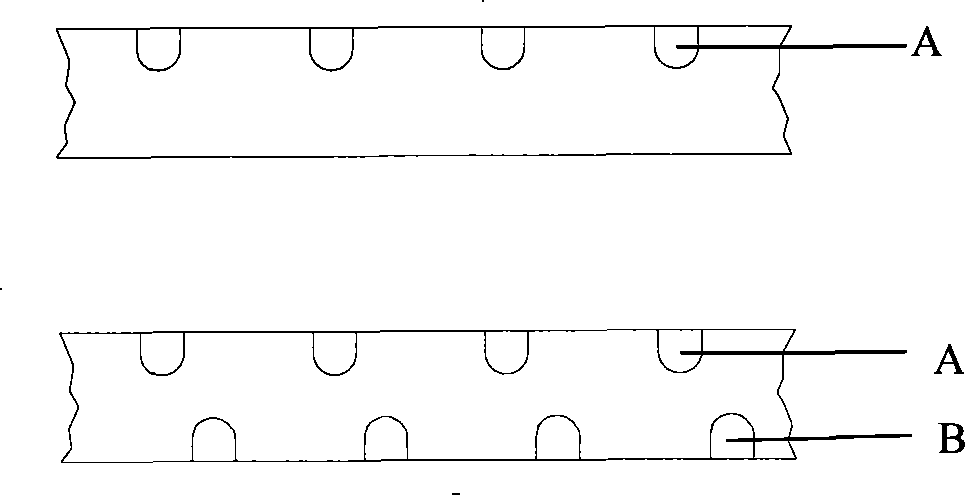
[0065] According to the set procedure, laser is used to drill holes on the surface of the bracket. The width of the hole on the outer surface of the bracket is 0.0003mm, and the depth is 0.0002mm.
[0066] Clean the above-mentioned treated stent with acetone solution with a concentration of 99.5%, and then ultrasonically clean it with distilled water at a frequency of 100khz for 10 minutes. Finally, place the cleaned stent in a dryer, set the temperature at 37°C, and take it out after drying for 30 minutes. Standby; use distilled water to prepare a hydrochloric acid solution with a concentration of 15%, soak the stent in the prepared solution, place it in...
Embodiment 3
[0068] Embodiment 3 Preparation of glycine-arginine-glycine-aspartic acid-serine-tyrosine scaffold
[0069] The 316L stainless steel vascular stent was cut, slag-removed, and polished, placed in 75% medical alcohol, cleaned with ultrasonic waves at a frequency of 100khz for 10 minutes, set the temperature at 40°C, dried for 40 minutes, and then taken out.
[0070] Glycine-arginine-glycine-aspartic acid-serine-tyrosine was prepared into a 5mg / ml aqueous solution, the above-mentioned 316L stainless steel vascular stent was placed on the place to be sprayed by ultrasonic spraying, and the nozzle and 316L stainless steel vascular stent Set up the electrostatic generator, the voltage of the electrostatic generator is 100V, the spraying flow rate is set to 1ml / min, and the spraying time of each circle is 1min. After a total of 3 spraying circles, take it out and store it at 4°C for a long time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com