Novel medicinal application of carboxyl aminotriazole and acceptable salts of the carboxyl aminotriazole
A technology of carboxytriazole and its use, which is applied in the field of new medical applications of carboxyamidotriazole and its available salts, and can solve the problems of few specific therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: The protective effect of drugs on ALS transgenic model animals
[0075] B6SJL-Tg(SOD1*G93A)1Gur / J transgenic mice, which overexpress the human SOD1 gene containing mutations identified in ALS patients, develop adult-onset, progressive animal degeneration. These transgenic mice are considered a model of the disease and have been used to test a number of strategies to delay disease progression and mortality.
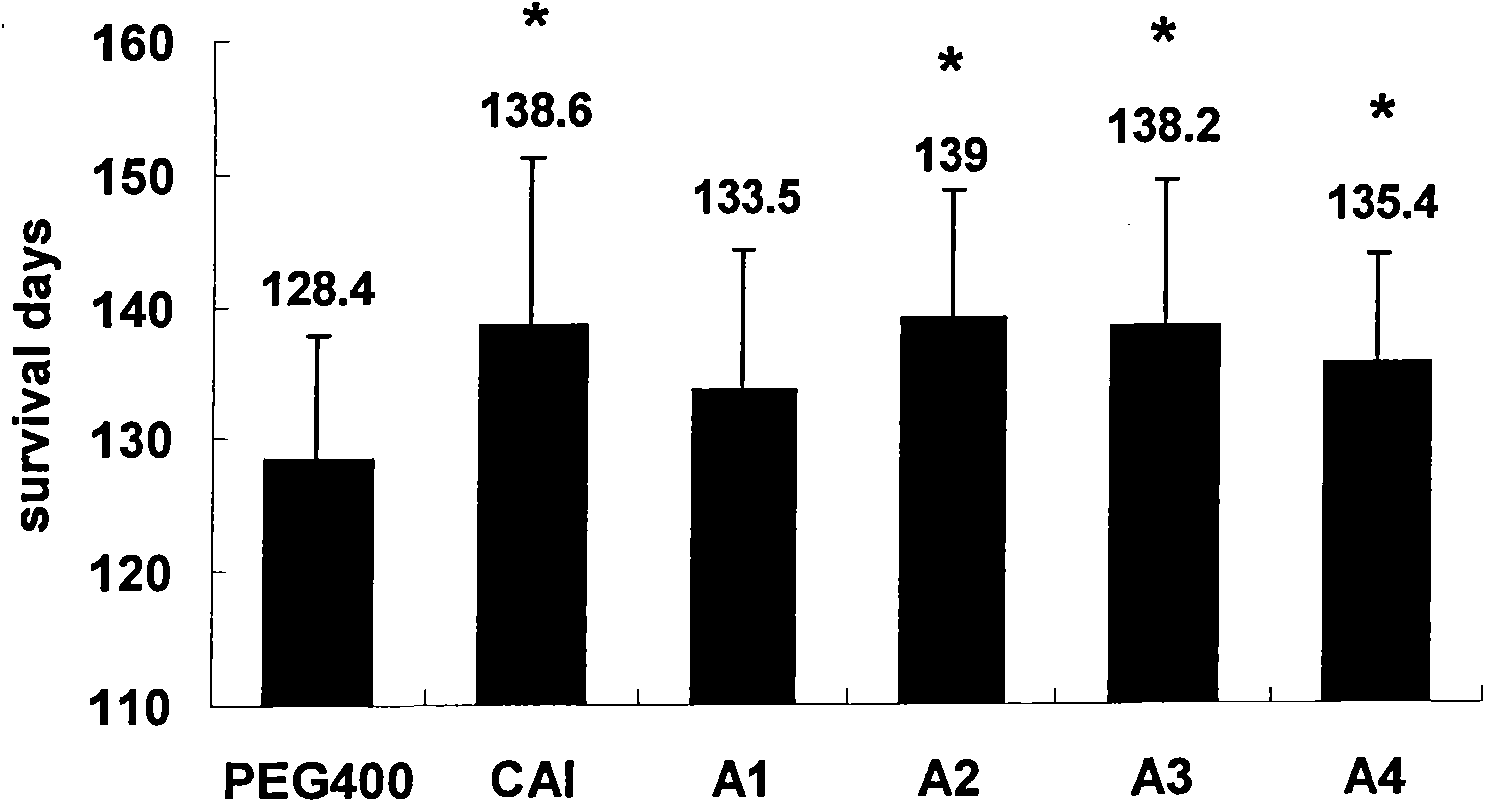
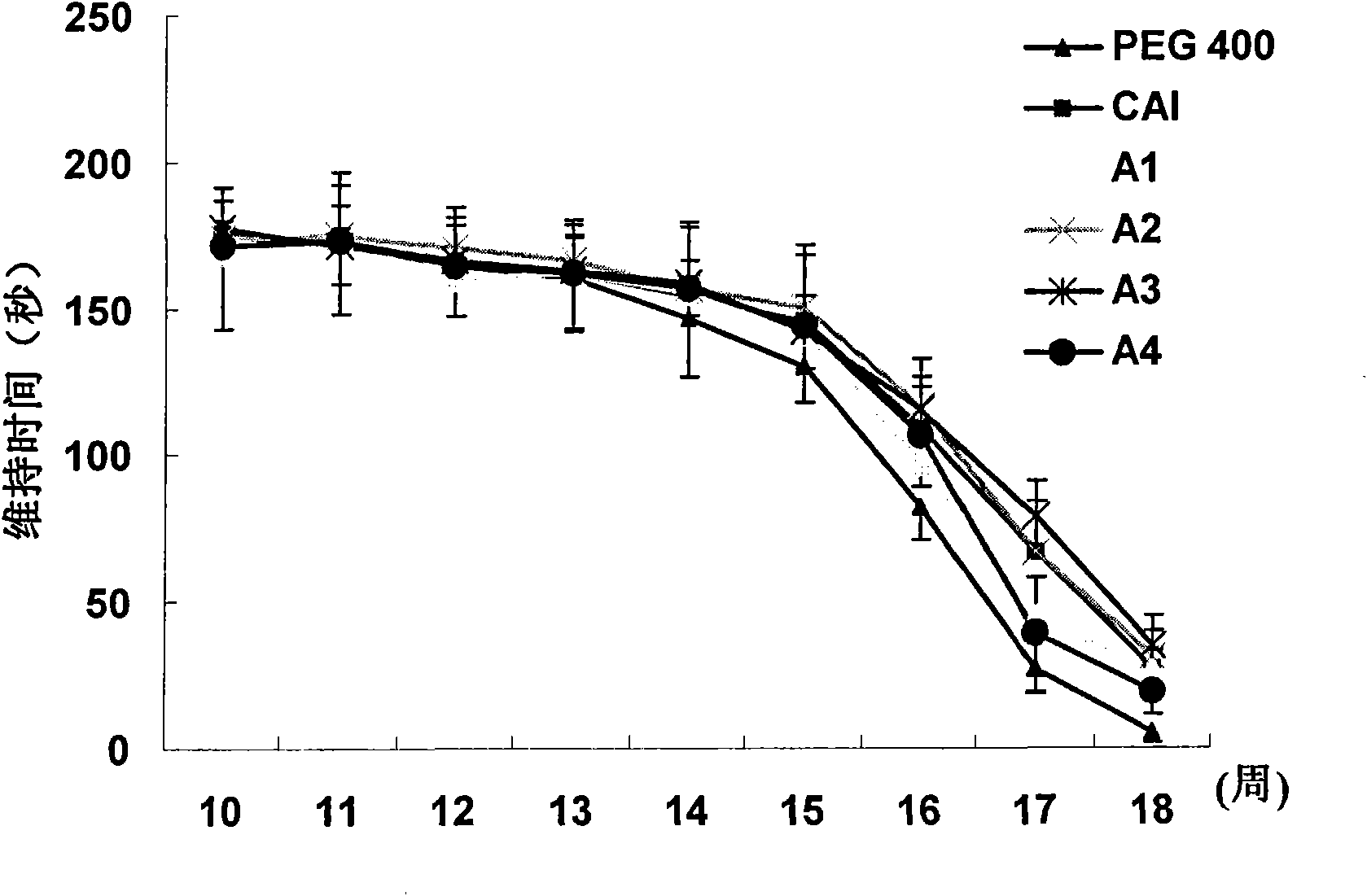
[0076] In order to evaluate the potential benefits of the present invention to ALS, the SOD1G93A transgenic mice of the control group and the administration group were orally administered with PEG400 solvent, CAI or various analogs of CAI (the compound of the structural formula is A, wherein R 1 is Cl or H, R 2 For Cl, H, Br, CH 3 , or CF 3 , R 3 Cl, Br, CF 3 , NO 2 or CN, X is O, S, or C=O). The four carboxyaminotriazole analogues used in this example are A1-A4 in Table 1, respectively, and the doses are all 20 mg / kg. Dosing began when mice were ...
Embodiment 2
[0082] Example 2: Carboxytriazole inhibits MPTP-induced oxidative stress
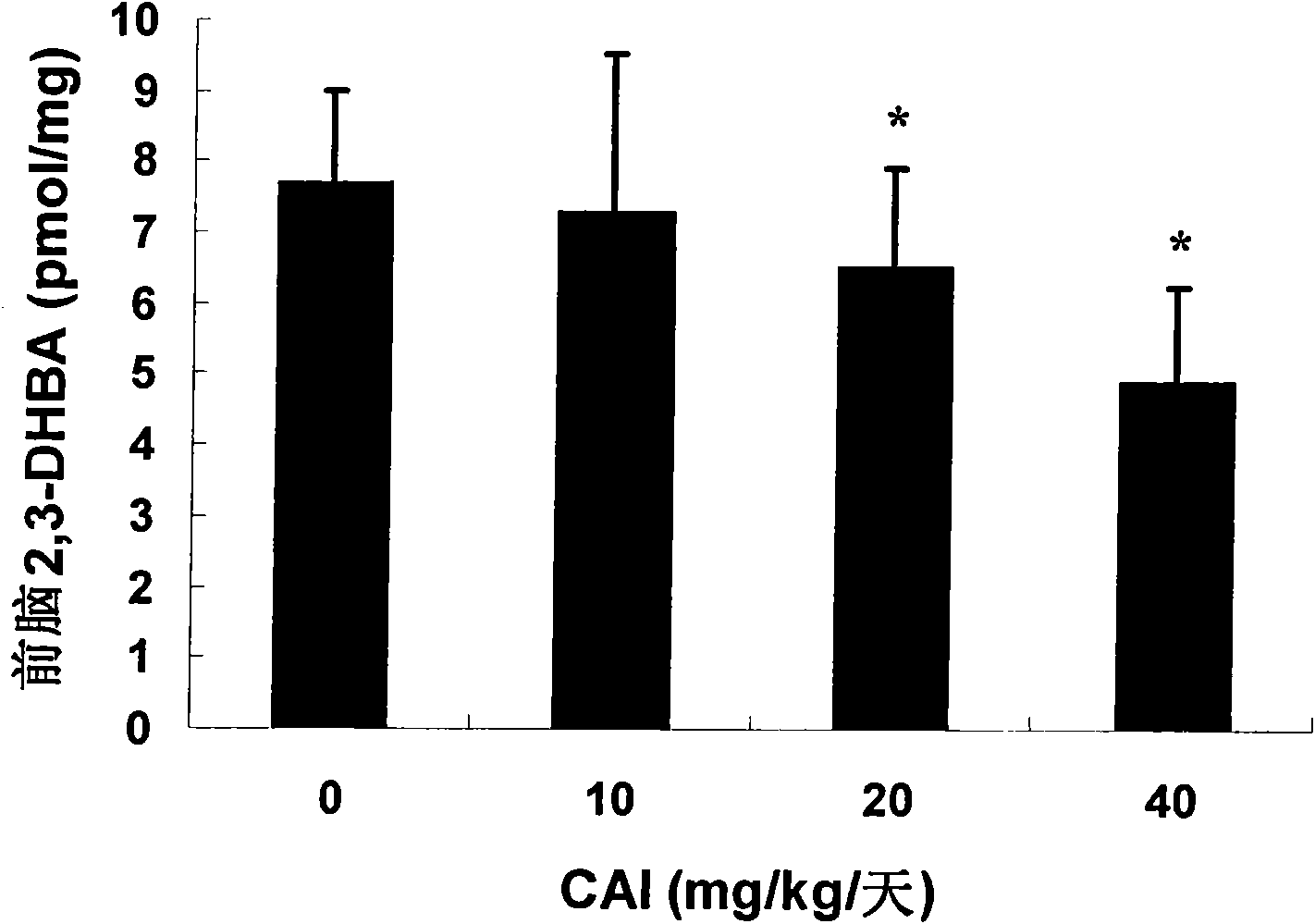
[0083] Male C57BL / 6 mice, weighing 18g-22g, 12 / group, were divided into 4 groups. Animals were given carboxytriazole by intragastric administration every day for 8 consecutive weeks, the doses were 0, 10, 20, or 40 mg / kg / day. On the day of the study, mice were subcutaneously injected with 30 mg / kg of the neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to increase oxidative stress in the animals' brains. irritating reaction. One hour later, the mice were intraperitoneally injected with 100 mg / kg of sodium salicylate. One hour after the injection, the animals were sacrificed, the forebrain was separated, and the content of 2,3-dihydroxybenzoic acid (2,3-DHBA) was determined.
[0084] Such as image 3 As shown, the results show that treatment with carboxytriazole at doses of 20 and 40 mg / kg / day can significantly reduce the forebrain oxidative stress produced by MPTP, thereby inhibiting n...
Embodiment 3
[0085] Example 3: Carboxytriazole analogues inhibit MPTP-induced oxidative stress
[0086] Male C57BL / 6 mice, body weight 18g-22g, 10 mice / group, divided into 11 groups. The animals were given the compounds A1-A10 in Table 1 by intragastric administration every day for 8 consecutive weeks, and the dose was 20 mg / kg / day. On the day of the study, mice were subcutaneously injected with 30 mg / kg of the neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to increase oxidative stress in the animals' brains. irritating reaction. One hour later, the mice were intraperitoneally injected with 100 mg / kg of sodium salicylate. One hour after the injection, the animals were sacrificed, the forebrain was separated, and the content of 2,3-dihydroxybenzoic acid (2,3-DHBA) was determined.
[0087] Such as Figure 4 As shown, the results showed that carboxylaminotriazole analogues such as A1-A10 could reduce the forebrain oxidative stress produced by MPTP to varying degrees, there...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com