Oral controlled release compositions comprising vitamin d compound and waxy carrier
A technology of vitamins and compounds, applied in the field of controlled-release pharmaceutical compositions, can solve the problems of early detection and treatment of hyperparathyroidism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 - Modified release formulation
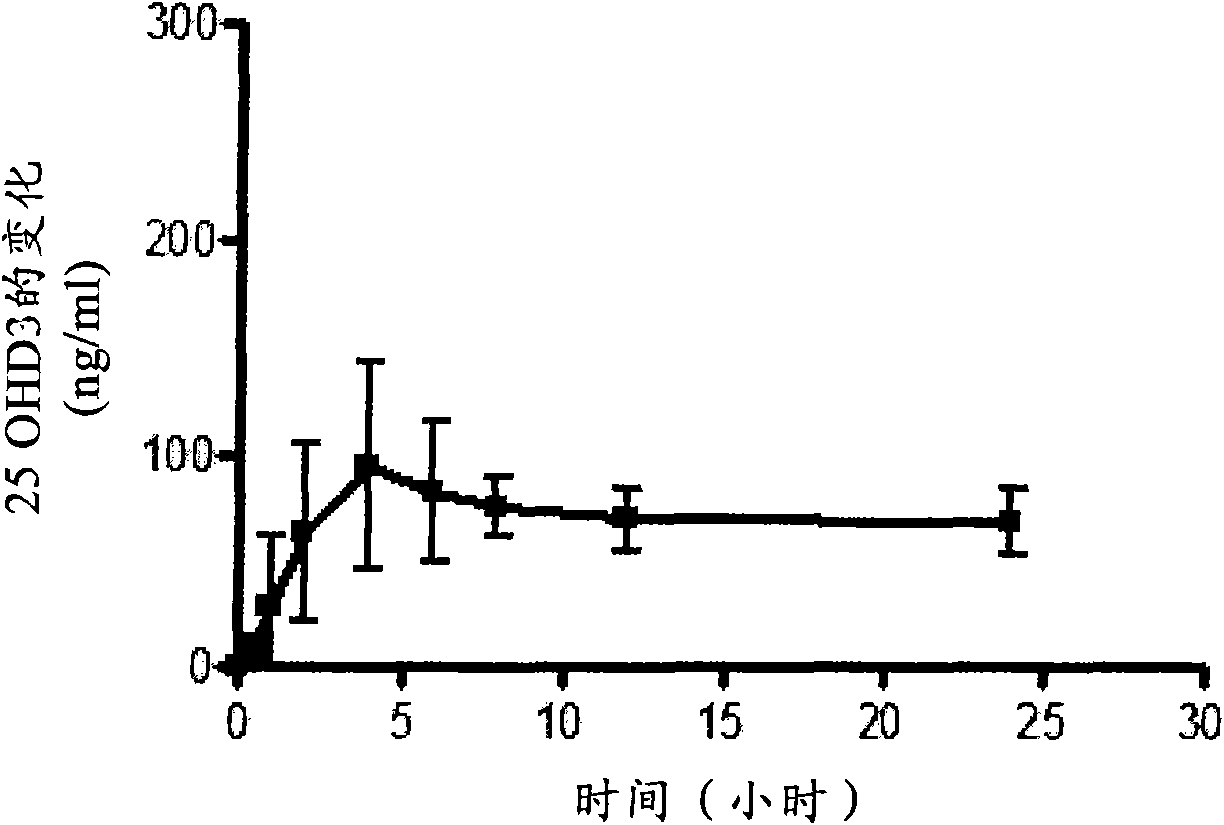
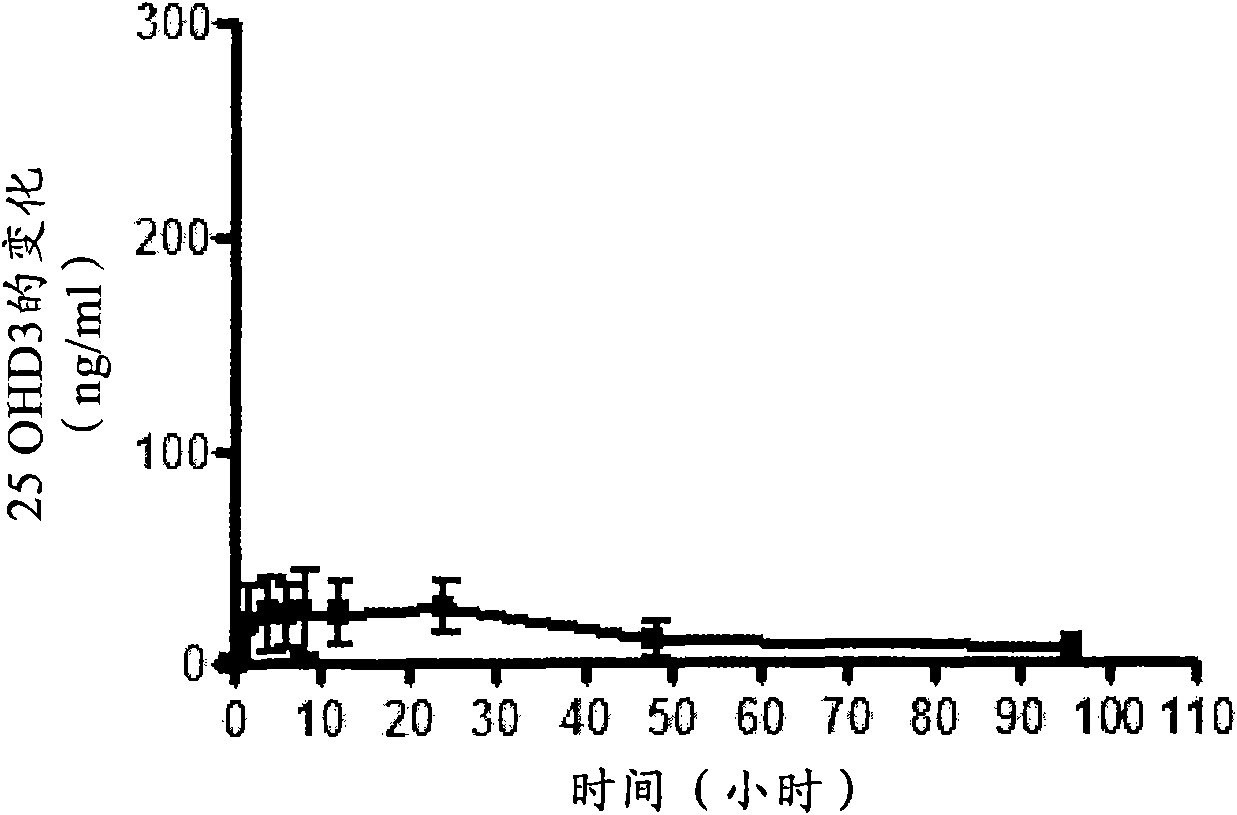
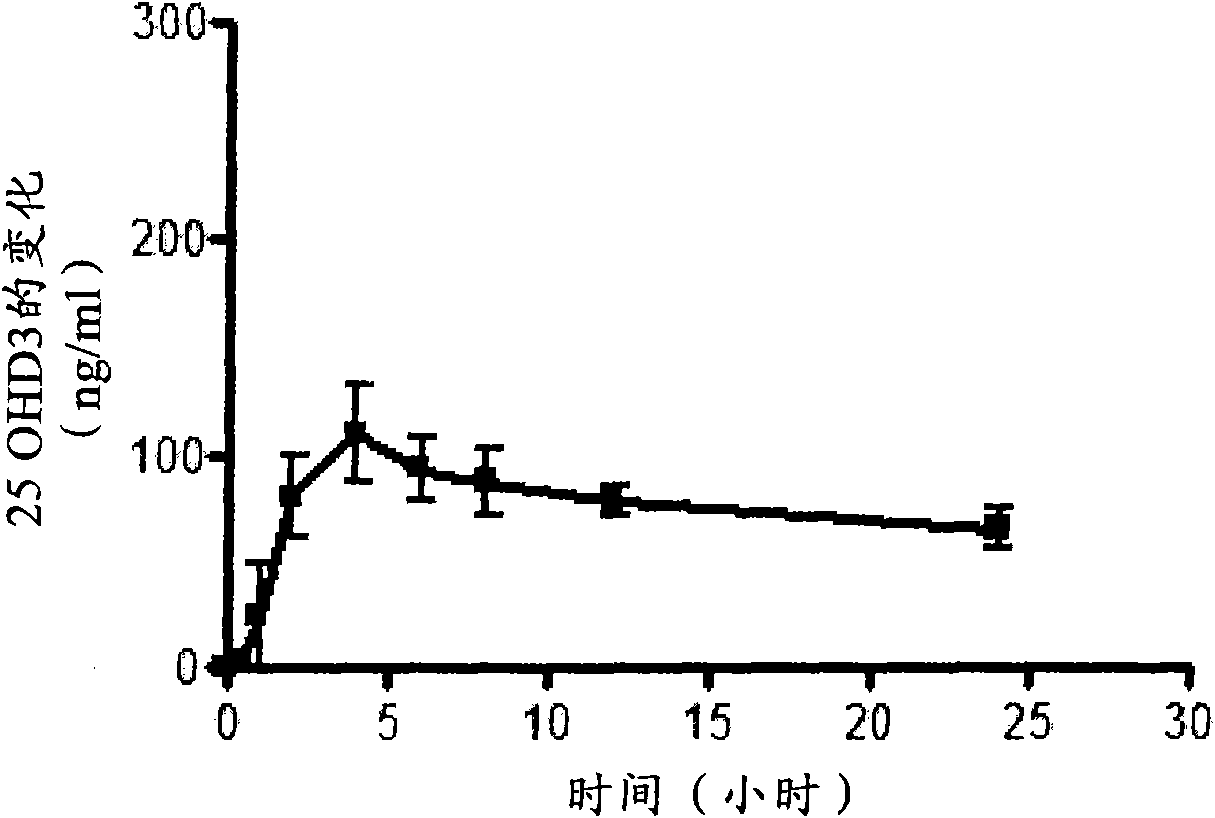
[0118] Nine oral vitamin D preparations were prepared according to Table 3 below by uniformly mixing the ingredients in the amounts listed in Table 3, and filling the mixture into hard capsules. Formulation 9 is an immediate release formulation according to the prior art, wherein MIGLYOL 812N is a trade name for caprylic / capric triglyceride, available from CONDEA Chemie GmbH of Cranford, New Jersey, USA. The formulation is formulated with the equivalent of 250 μg of 25-hydroxyvitamin D 3 A single dose of Yucatan minipigs (approximately 10 kg) was administered to groups consisting of 5 animals per group. Equivalent 250 μg 25-hydroxyvitamin D 3 A tenth group of five Yucatan minipigs was administered by intravenous bolus.
[0119]
[0120] Blood was collected before dosing, and at 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 96, 168, 240, 336, 432, 504, 576, and 672 hours after dosing. Serum 25-hydroxyvitamin D 3 Levels were detected b...
Embodiment 2
[0131] Example 2-Pharmacokinetic study of oral capsules in minipigs
[0132] The aim of the study was to evaluate 25-hydroxyvitamin D in male Yucatan pigs (-45kg body weight) 3 systemic absorption, administration is carried out as follows: a) 1×250 μg 25-hydroxyvitamin D 3 Modified release (MR) capsules; b) 2×250 μg MR capsules; c) 4×250 μg MR capsules; d) 1×1000 μg MR capsules; e) 1×250 μg 25-hydroxyvitamin D 3 Immediate release (IR) capsules; and f) 1 x 250 μg MR capsules administered for 3 consecutive days.
[0133] The MR formulation was prepared based on the formulation of Example 1, Group 7 above. Higher concentration of 25-hydroxyvitamin D in the case of 1000 μg MR capsules 3 offset by the relative reduction in ethanol.
[0134] To prepare IR preparations, 25-hydroxyvitamin D 3 (0.12% wt / wt; 250 μg per capsule) dissolved in ethanol USP (2.32% wt / wt; solublizer), mixed with corn oil USP (97.54% / wt, antioxidant) mixed. The corn oil solution (205 mg) was filled int...
Embodiment 3
[0150] Example 3 - Oral Capsule Minipig Systemic Exposure Study
[0151] The aim of this study was to assess systemic 25-hydroxyvitamin D in healthy normal male Yucatan pigs (body weight -50-60kg) maintained on a diet including adequate vitamin D intake, administered daily for 21 days 3 Concentration increases, administered as follows: a) 25 μg immediate release (IR) 25-hydroxyvitamin D 3 Capsules (group 1), b) 25 μg modified-release (MR) 25-hydroxyvitamin D 3 capsules (group 2), and c) 125 μg MR 25-hydroxyvitamin D 3 capsules (group 3).
[0152] The MR formulation was prepared based on the formulation of Example 1, Group 7 above. 25 hydroxyvitamin D 3 Differences in concentrations are offset by relative changes in ethanol.
[0153] For the preparation of IR formulations, 25-hydroxyvitamin D 3 (0.12% wt / wt; 250 μg per capsule) dissolved in ethanol USP (2.32% wt / wt; solublizer) and mixed with corn oil USP (97.54% / wt, antioxidant) mixed. Corn oil solution (205 mg) was f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| kinematic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com