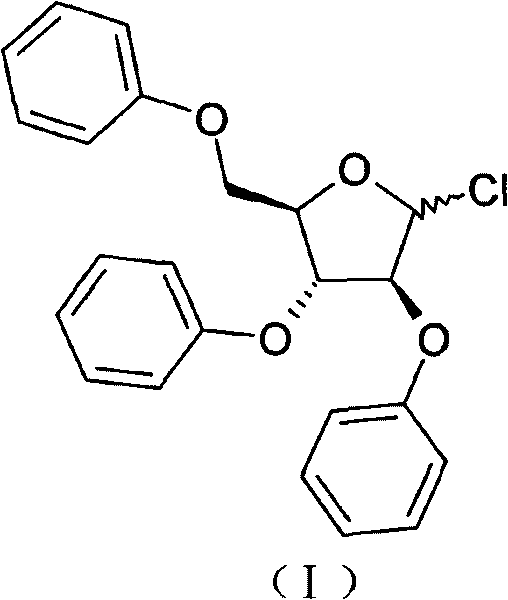
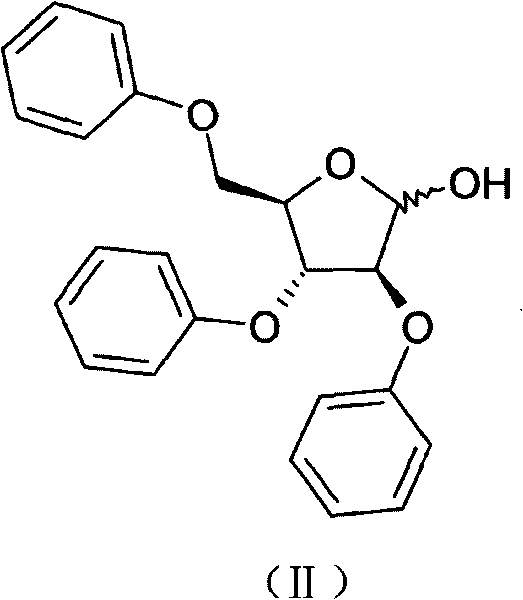
Method for preparing 2,3,5-tri-O-tribenzyl-1-chloro-D-arabinofuranose
A technology of arabinofuranose and benzyl, which is applied in the field of preparation of 2,3,5-tri-O-tribenzyl-1-chloro-D-arabinofuranose, which can solve the problem of increased man-hours, decreased yield, and material consumption and increased energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of 2,3,5-tri-O-benzyl-1-chloro-D-arabinofuranose
[0019] Step 1: 38g (0.1mol) of 2,3,5-tri-O-benzyl-D-arabinofuranose and 200ml of trichloroacetonitrile were placed in a 500ml three-necked flask, and dry hydrogen chloride gas was introduced, heated to reflux, and reacted After 2 hours, the spots of the reactants detected by TLC basically disappeared (toluene / methanol 9:1). Trichloroacetonitrile was recovered under reduced pressure to dryness to obtain 43.0 g of light yellow oil.
[0020] Step 2: Take the oil in the previous step, add 300ml of ethyl acetate, heat to dissolve, add 0.5g of activated carbon, heat to reflux for 20min, filter while it is hot, and after the filtrate is cooled to room temperature, solids begin to precipitate slowly, then use an ice-water bath to dissolve It was cooled to below 0°C, kept for 4 hours, filtered to obtain a white solid, and dried in vacuum at 50°C to obtain 36.5 g of 2,3,5-tri-O-benzyl-1-chloro-D-arabinofuranose (cont...
Embodiment 2
[0022] Preparation of 2,3,5-tri-O-benzyl-1-chloro-D-arabinofuranose
[0023] Step 1: 38g (0.1mol) of 2,3,5-tri-O-benzyl-D-arabinofuranose, 200ml of dichloromethane, placed in a 500ml three-necked flask, cooled in an ice-water bath to below 5°C, and dichloromethane was added dropwise 12.3ml of sulfoxide was added dropwise within 2 hours, and heated to reflux. After 12 hours of reaction, the spots of the reactant basically disappeared as detected by TLC (toluene / methanol 9:1). Return to dichloromethane under reduced pressure and collect to dryness to obtain 45.6 g of light yellow oil.
[0024] Step 2: Take the oil in the previous step, add 300ml of ethyl acetate, heat to dissolve, add 0.5g of activated carbon, heat to reflux for 20min, filter while it is hot, and after the filtrate is cooled to room temperature, solids begin to precipitate slowly, then use an ice-water bath to dissolve It was cooled to below 0°C, kept for 4 hours, filtered to obtain a white solid, and dried in ...
Embodiment 3
[0026] Preparation of 2,3,5-tri-O-benzyl-1-chloro-D-arabinofuranose
[0027] Step 1: 38g (0.1mol) of 2,3,5-tri-O-benzyl-D-arabinofuranose and 200ml of dichloroethane were placed in a 500ml three-necked flask, cooled in an ice-water bath to below 5°C, and two 12.3ml of thionyl chloride was added dropwise within 2 hours, heated to 80°C, stirred for 2.5 hours, and the spots of the reactant basically disappeared as detected by TLC (toluene / methanol 9:1). Return to dichloromethane under reduced pressure and collect to dryness to obtain 44.6 g of light yellow oil.
[0028] Step 2: Take the oil in the previous step, add 300ml of ethyl acetate, heat to dissolve, add 0.5g of activated carbon, heat to reflux for 20min, filter while it is hot, and after the filtrate is cooled to room temperature, solids begin to precipitate slowly, then use an ice-water bath to dissolve It was cooled to below 0°C, kept for 4 hours, filtered to obtain a white solid, and dried in vacuum at 50°C to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com