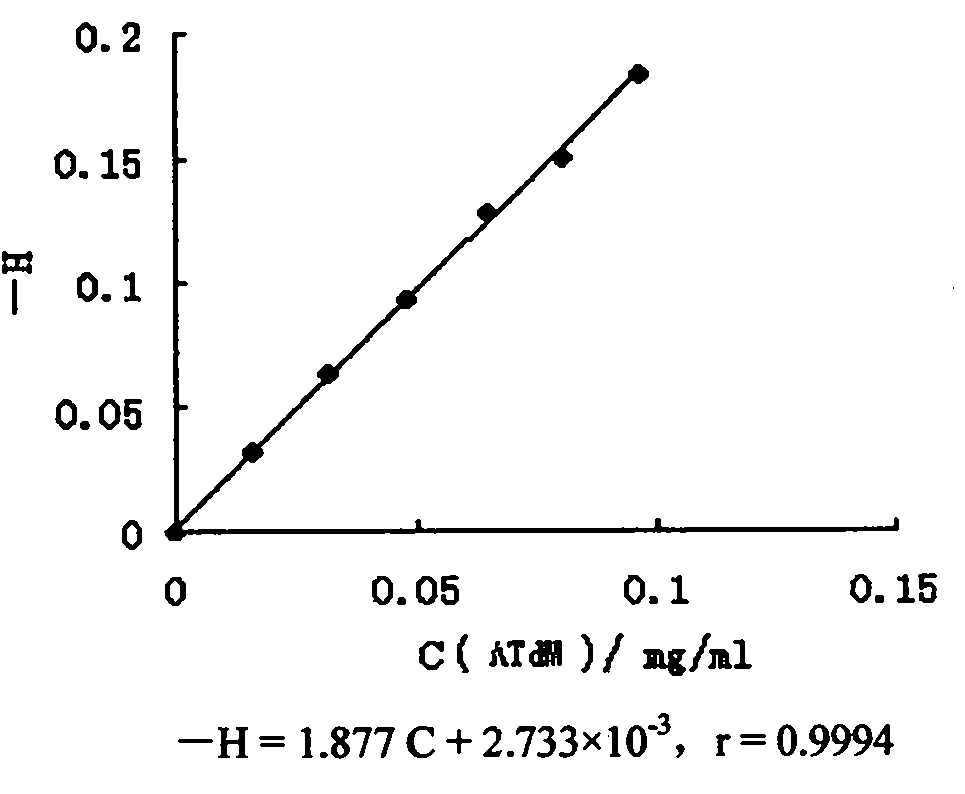Ancitabine dimyristate acid ester liposome and preparation method thereof
A technology of myristate and cyclocytidine, applied in the field of medicine, can solve the problems of poor water solubility, difficult administration, difficult absorption, etc., and achieves the effects of high encapsulation rate, improved absorption, and improved curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0010] (1) Preparation
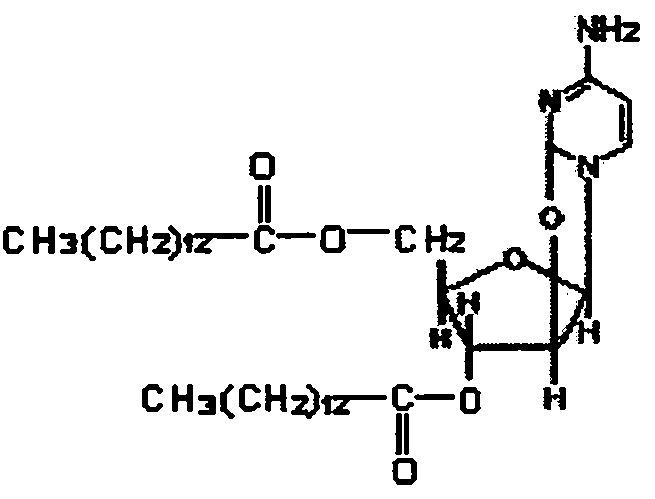
[0011] Cyclocytidine dimyristate liposomes were prepared by reverse-phase evaporation. Natural lecithin is separated by alumina column chromatography to obtain pure product, lecithin: cholesterol: distearoyl phosphatidylcholine is placed in a chicken heart bottle according to the molar ratio of 1:1:0.5, and an appropriate amount of chloroform is added to dissolve it . Accurately weigh the required amount of cyclocytidine dimyristate, add it into the same chicken heart bottle to dissolve it, remove chloroform by rotary evaporation under reduced pressure in a nitrogen atmosphere at 45°C, and form a dry film on the inner wall of the chicken heart bottle, diethyl ether and pH A phosphate buffer solution with a value of 7.15 was added in a volume ratio of 3:1 (add ether first to dissolve the lipid film, and then add a phosphate buffer solution), and after 20 minutes of ultrasonic dispersion by an ultrasonic cleaner, the resulting mixture was evaporated in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com