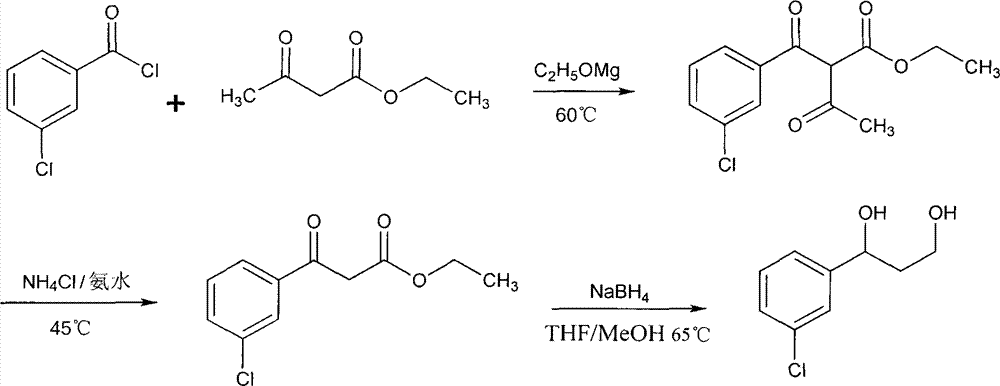
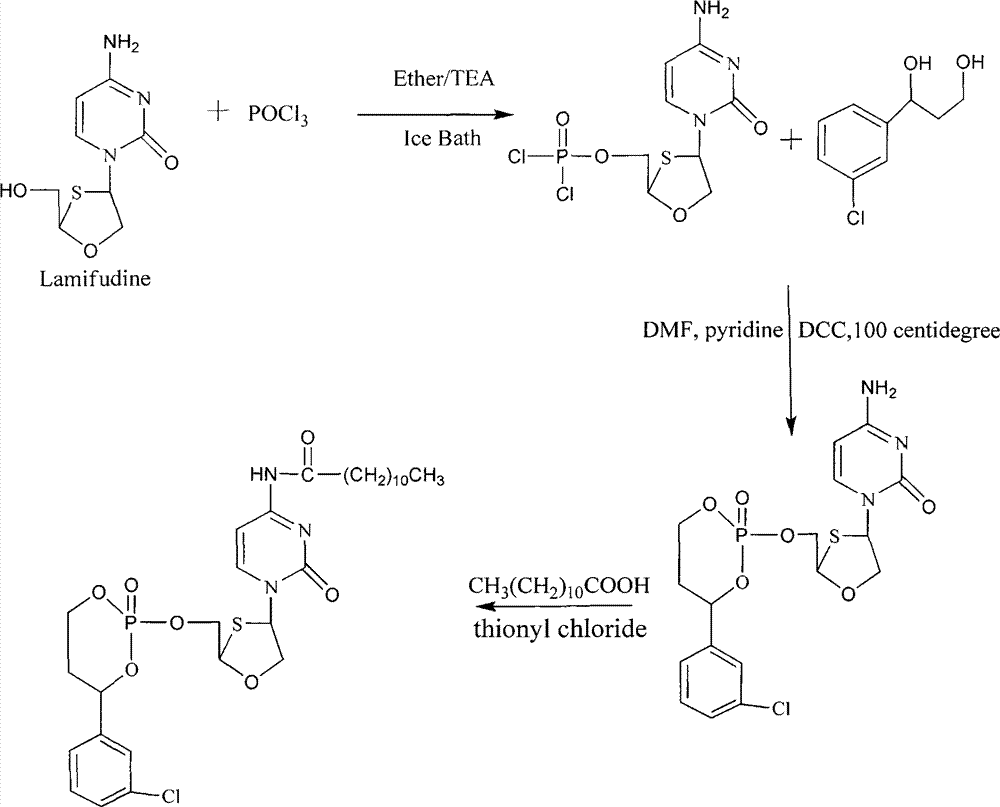
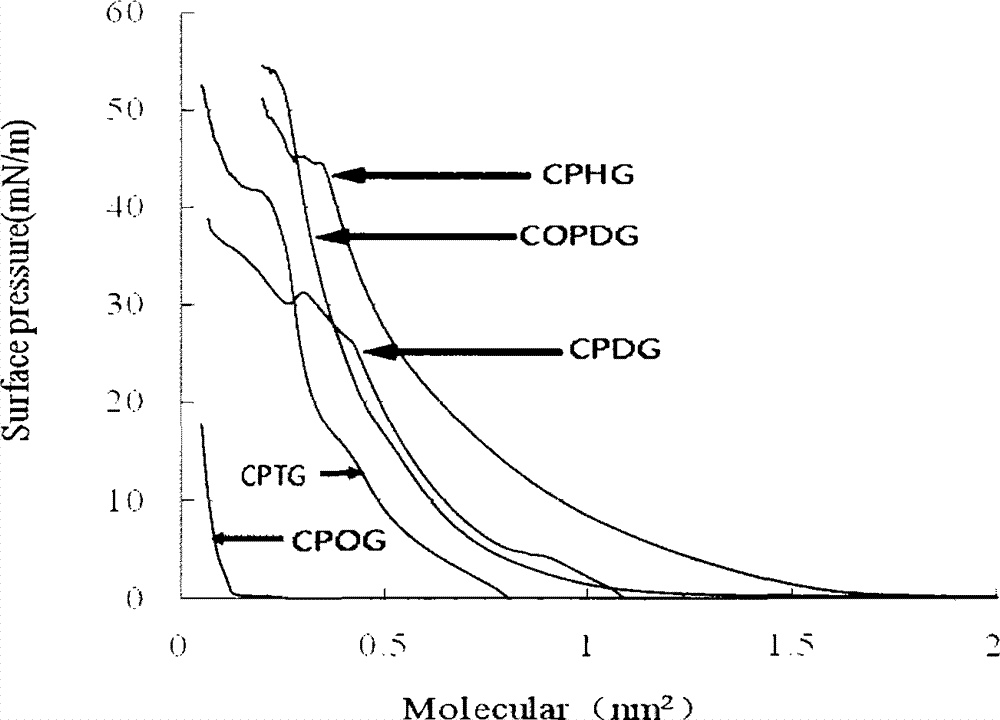
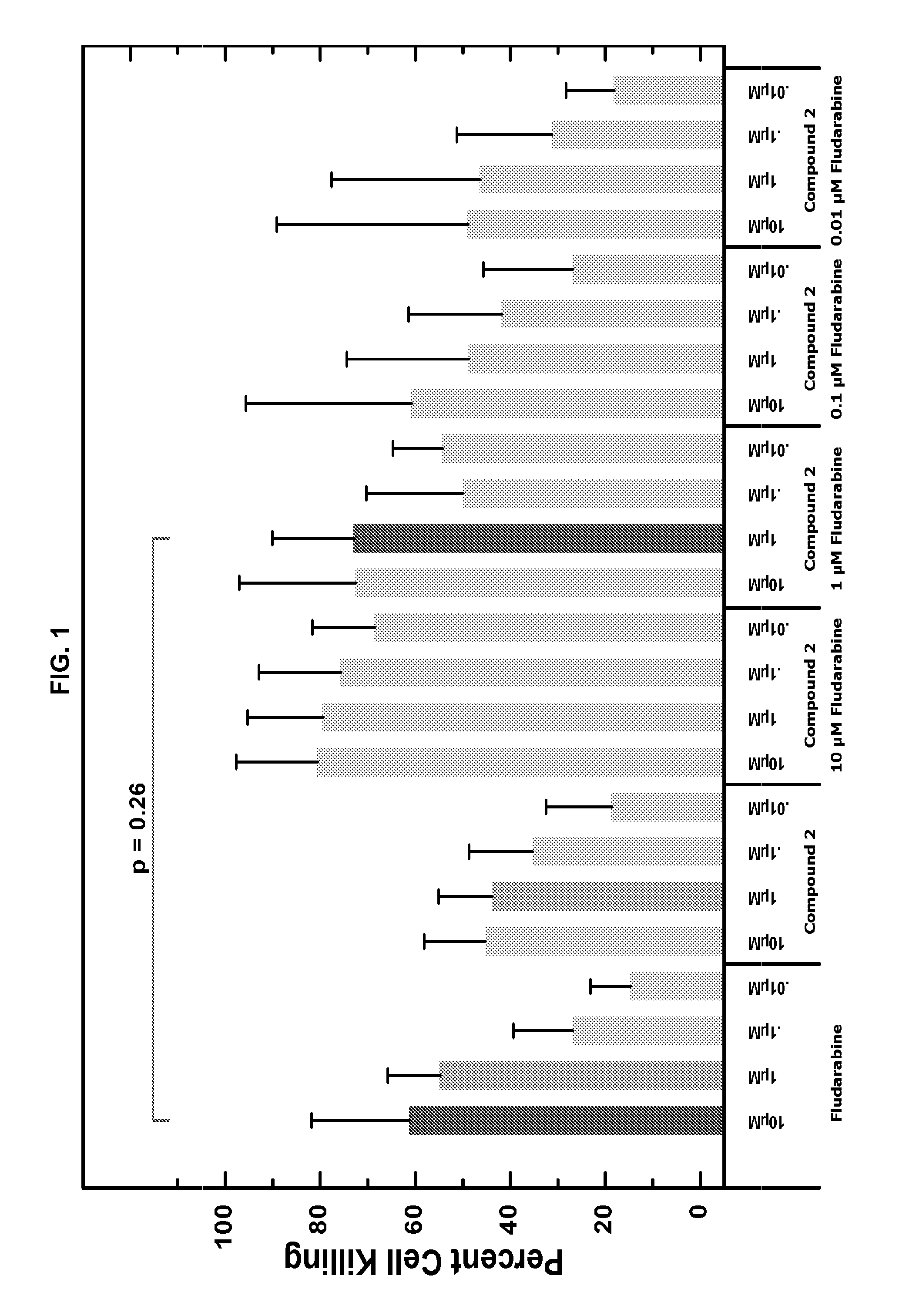
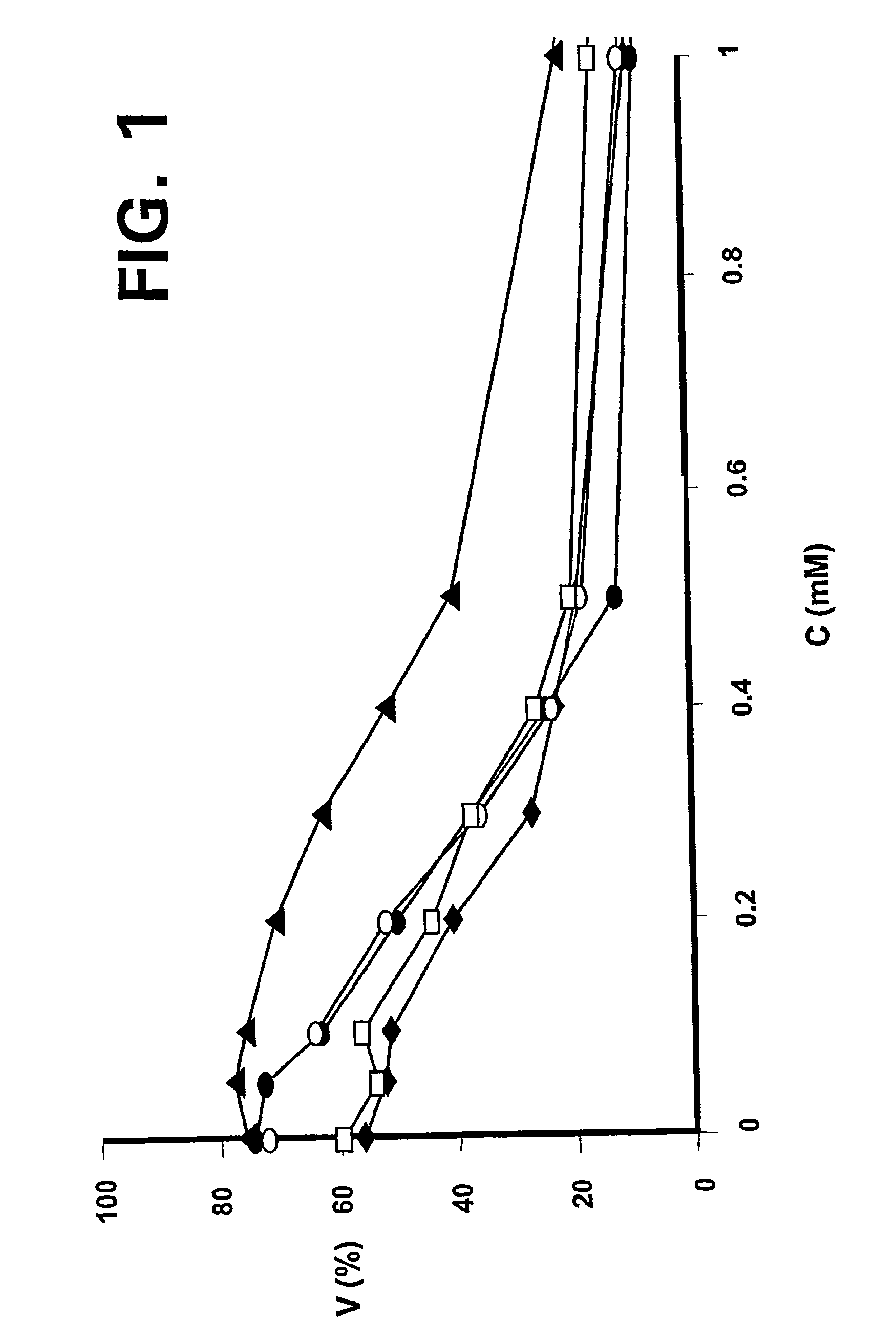
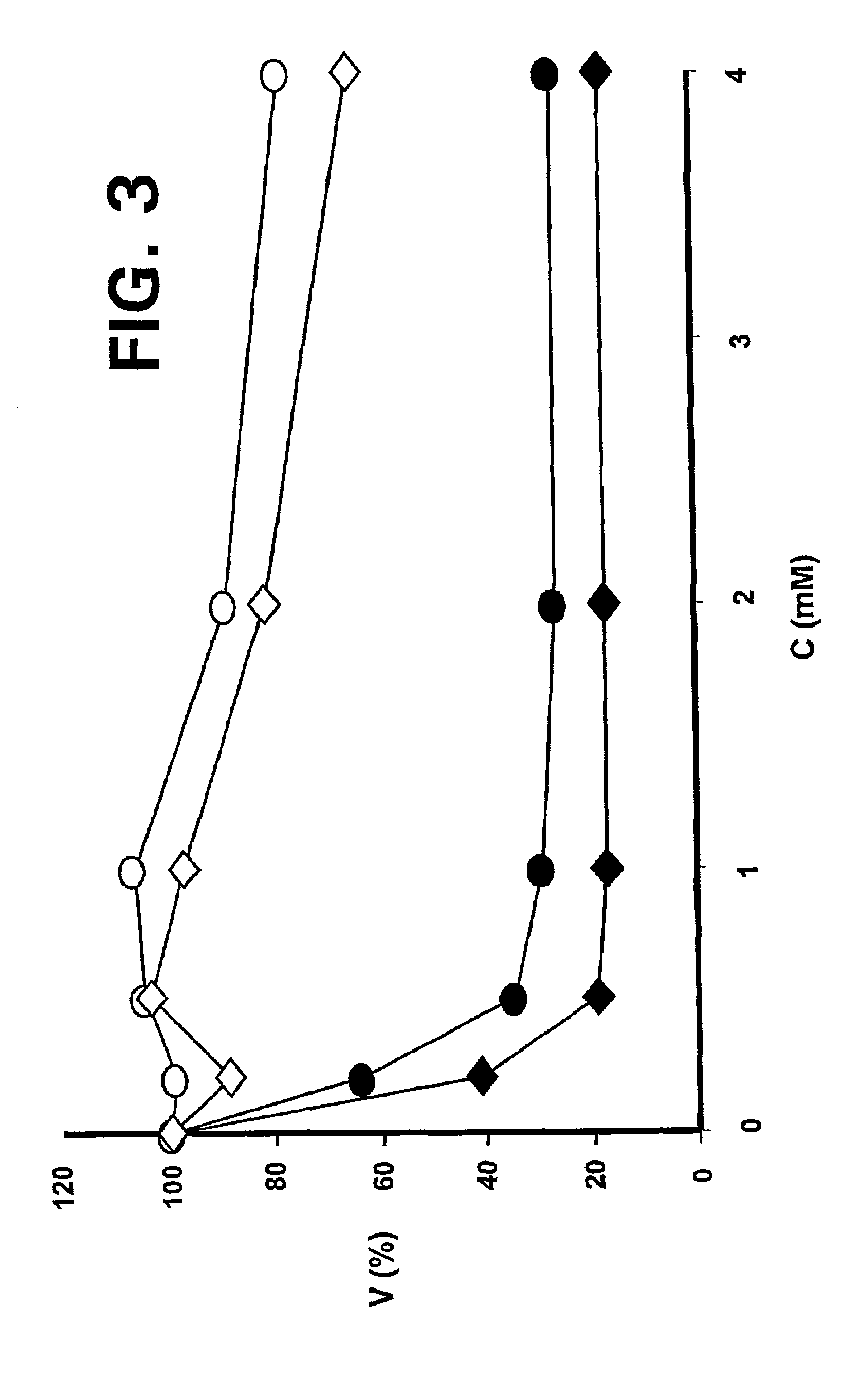
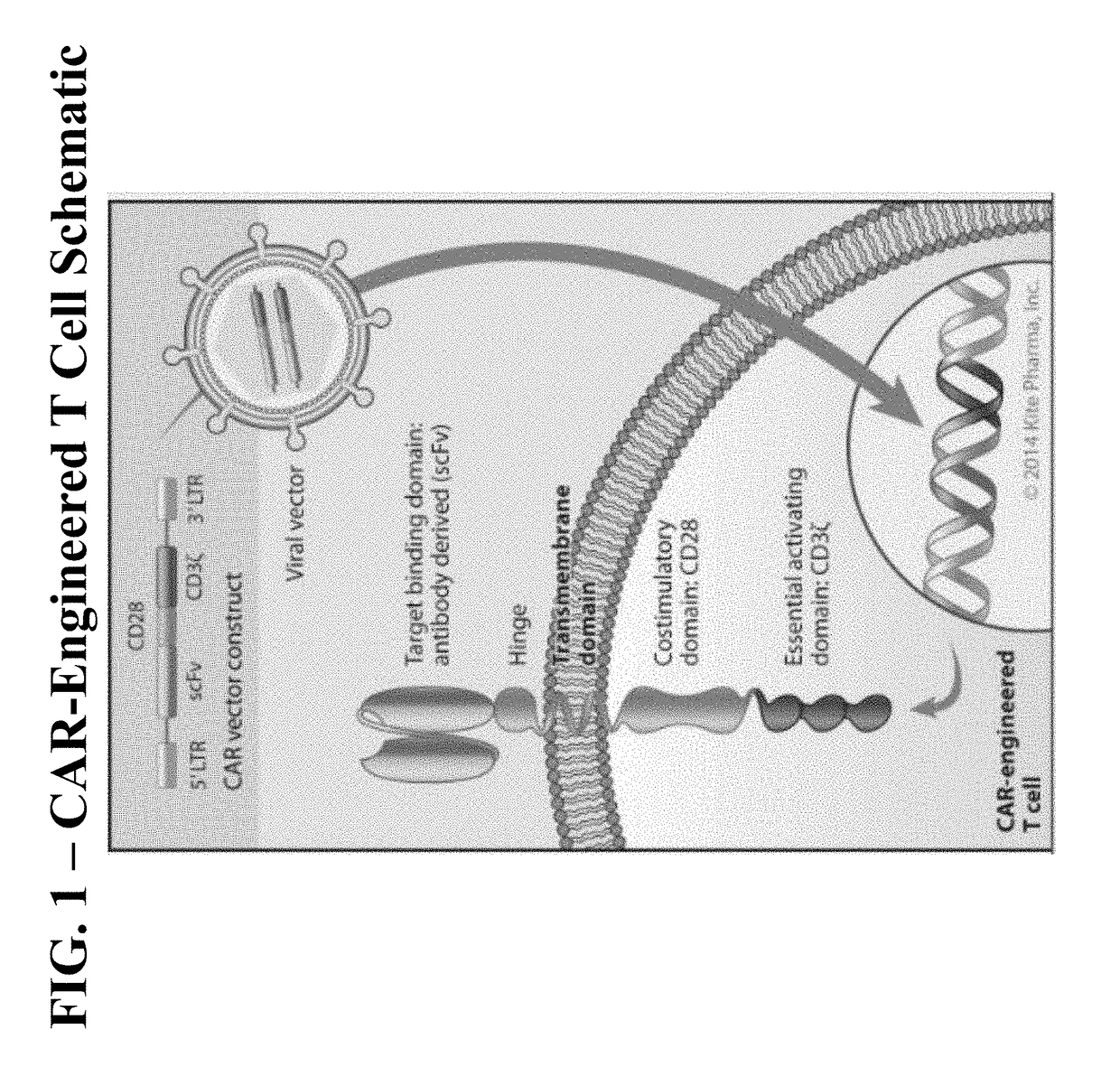
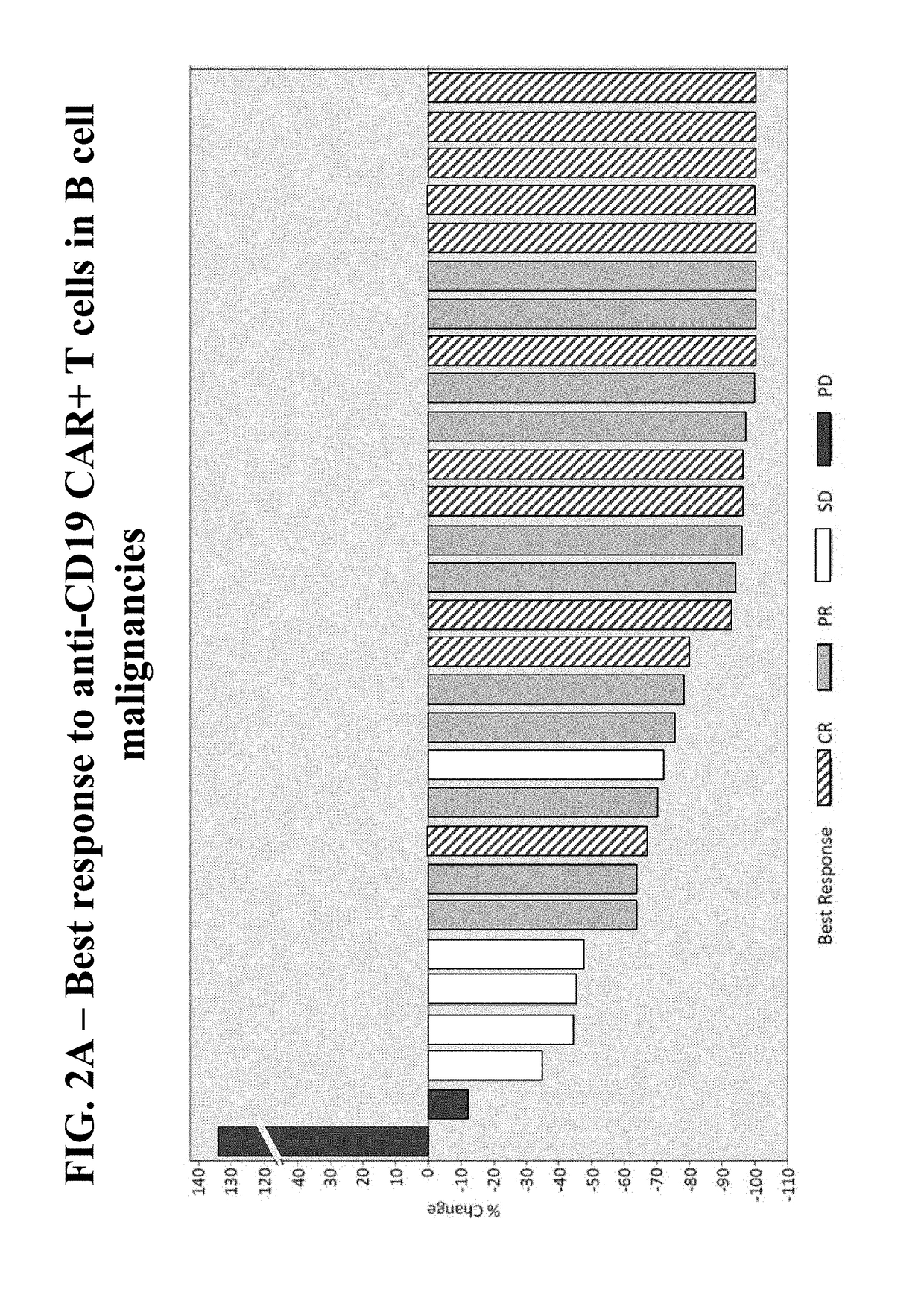
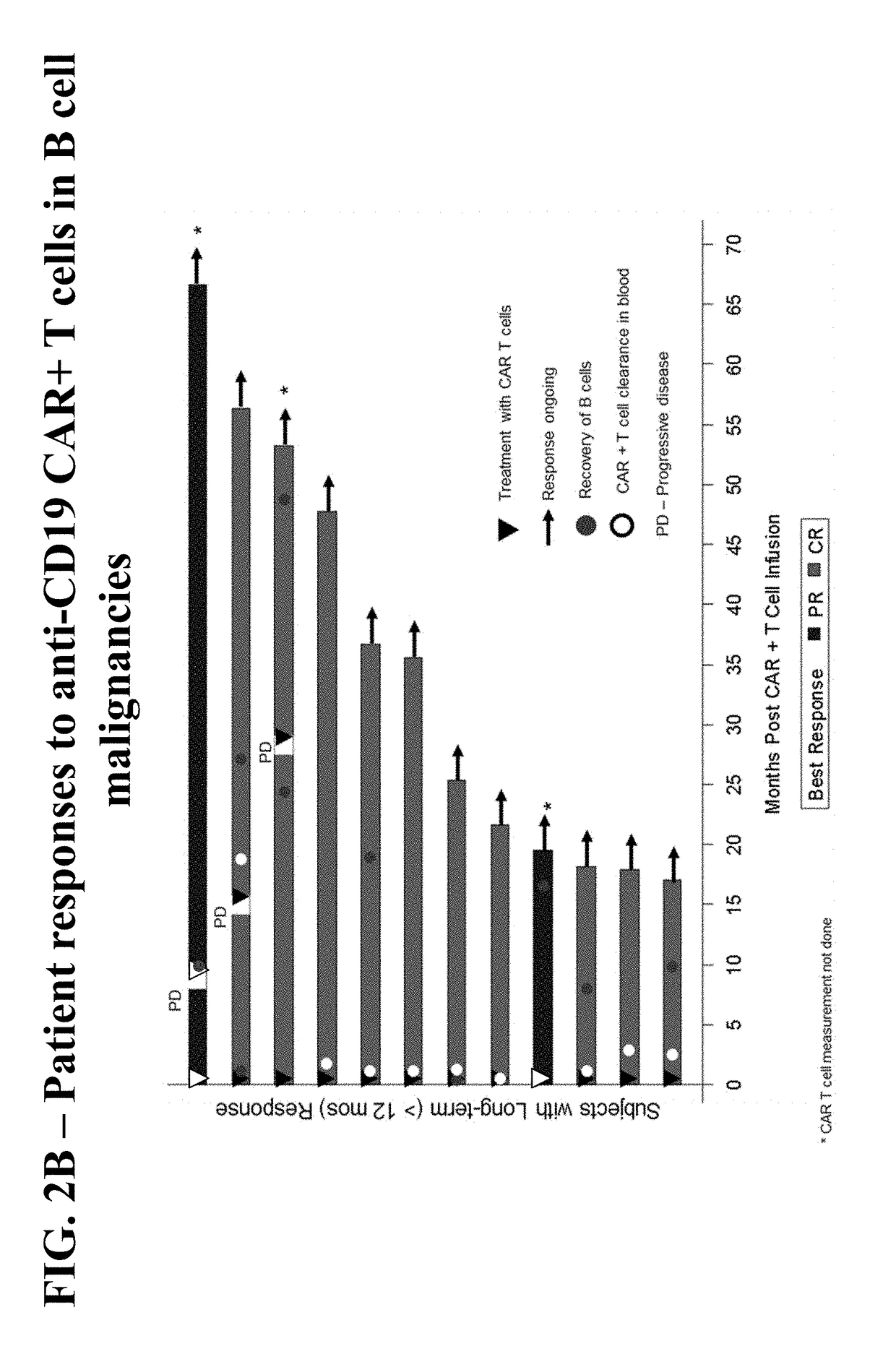
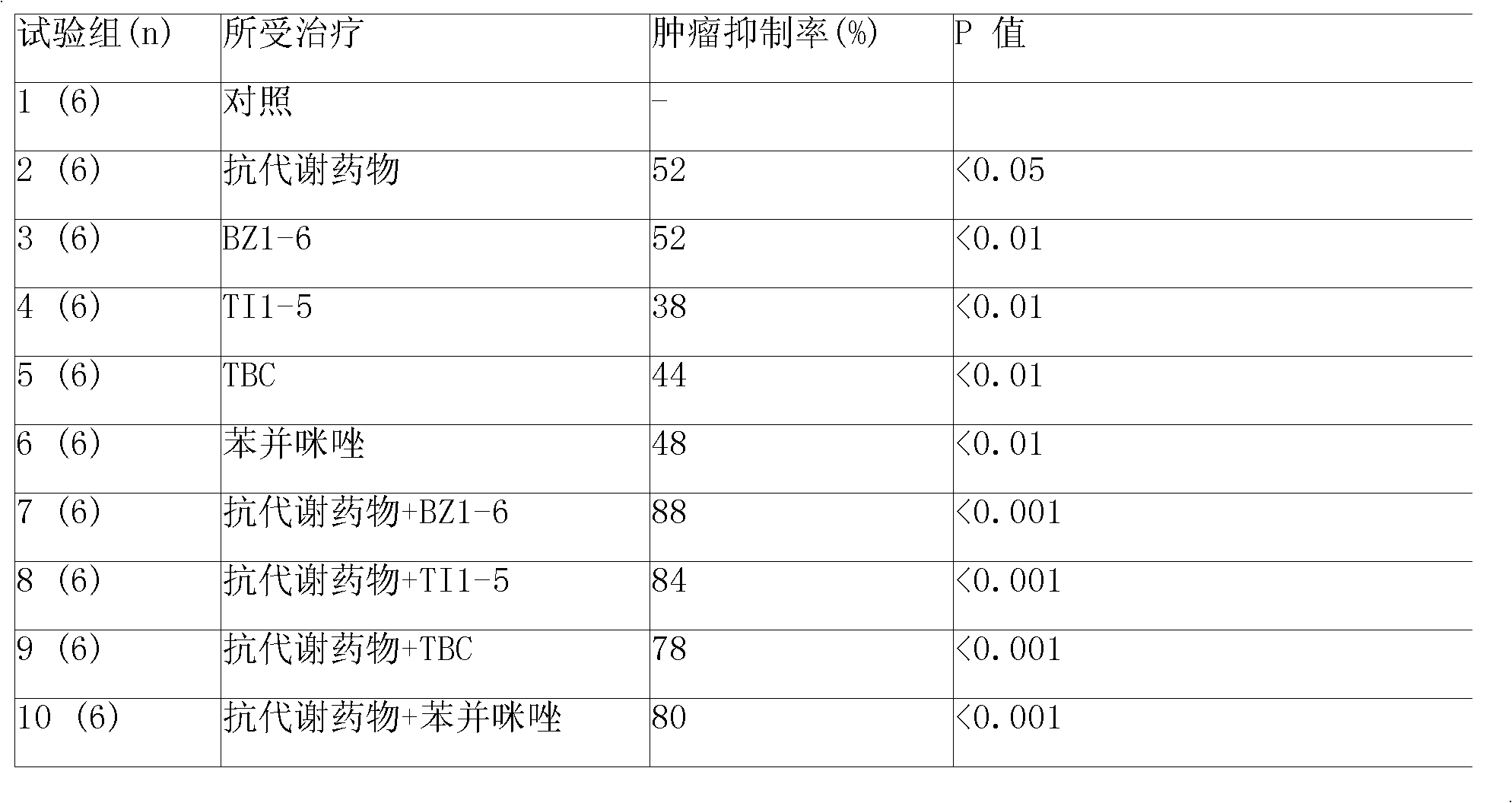
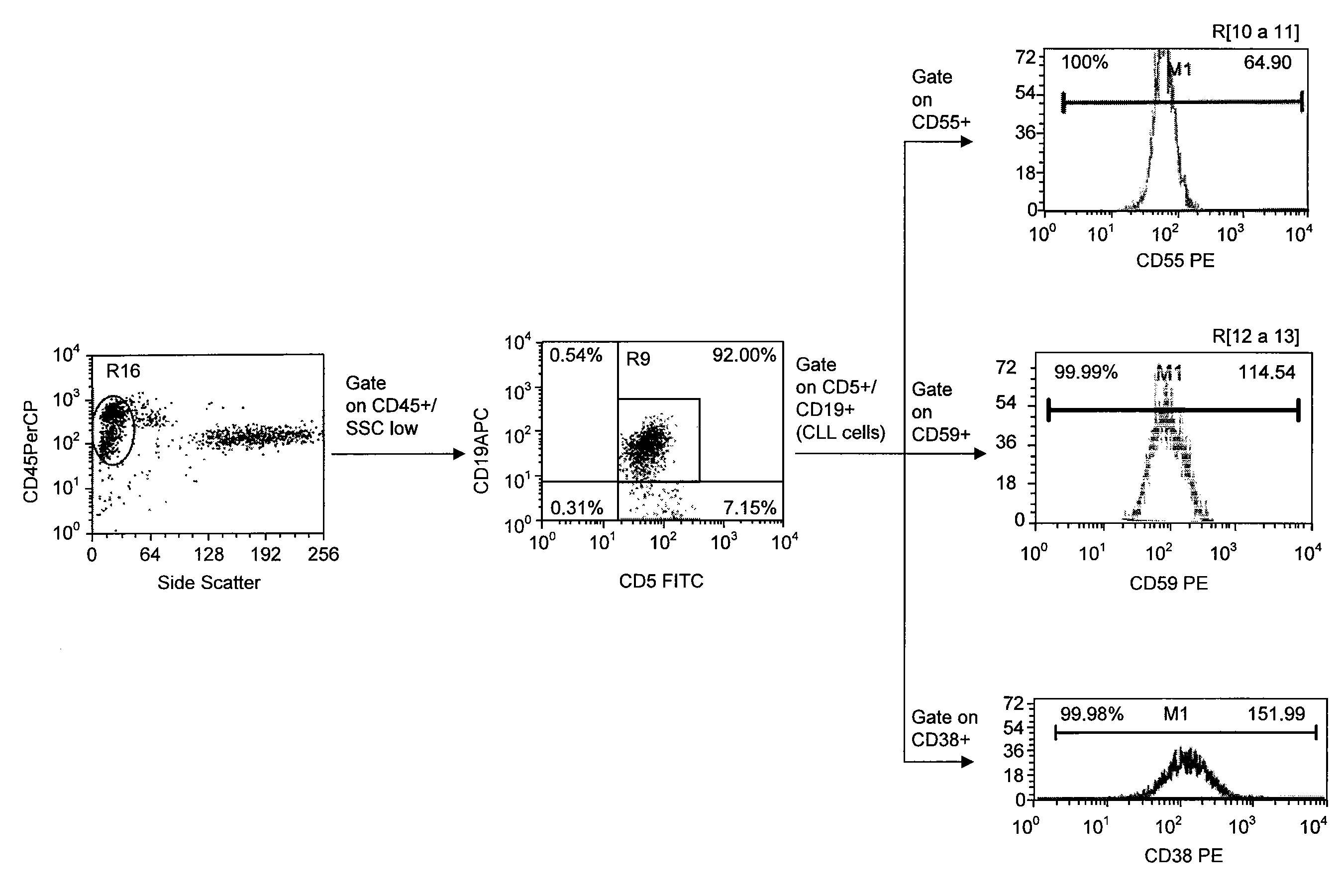
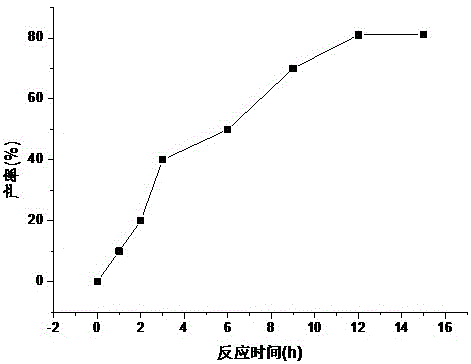
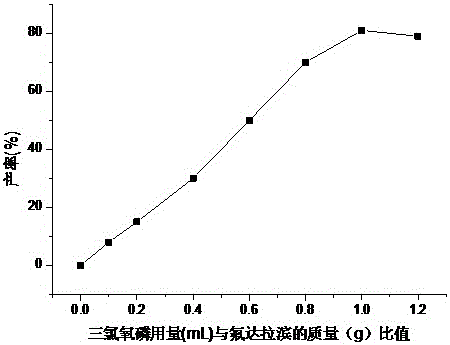
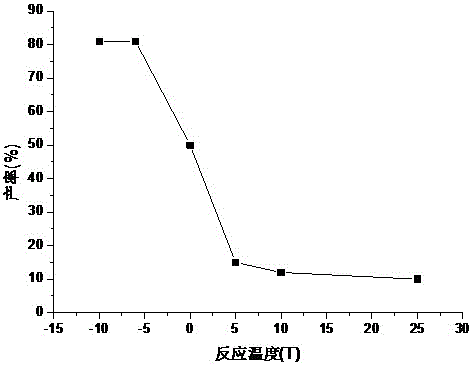
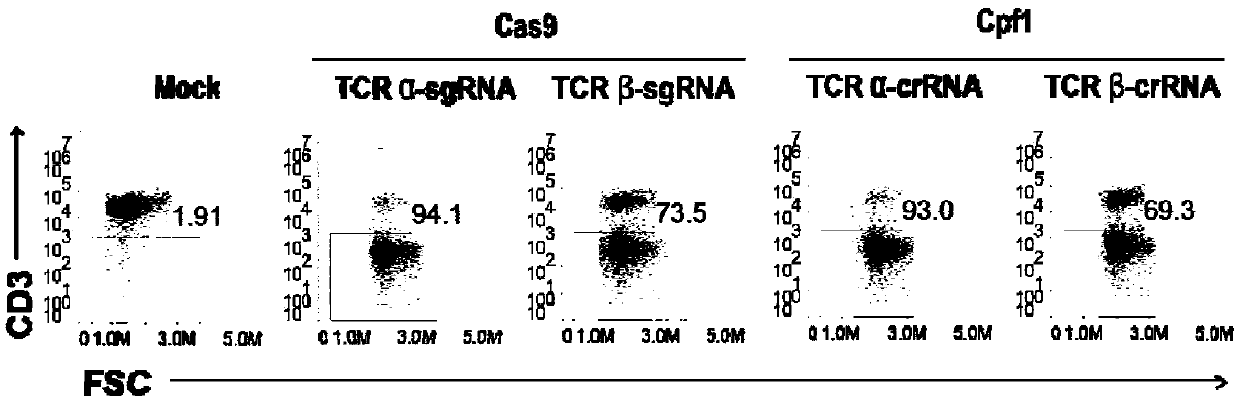
Patents
Literature
40 results about "Cyclophosphamide/fludarabine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fludarabine is highly effective in the treatment of chronic lymphocytic leukemia, producing higher response rates than alkylating agents such as chlorambucil alone. Fludarabine is used in various combinations with cyclophosphamide, mitoxantrone, dexamethasone and rituximab in the treatment of indolent non-Hodgkins lymphomas.
Topical composition for treatment of skin disorders
InactiveUS20070142317A1Shelf life stableNon toxicBiocideFlow mixersDiseaseAdenosine Deaminase Inhibitor
The present invention provides for a topical composition that includes a topical carrier and an adenosine deaminase inhibitor. Suitable specific adenosine deaminase inhibitors include, e.g., deoxycoformycin (dCF), deoxyadenosine (dAdo), cladrabine (CdA), fludarabine (F-Ara-A), cytrabine (Ara-C), and thioguanine. The present invention also provides for a method to treat lymphocyte mediated skin diseases and to alleviate symptoms associated with such skin diseases. The method includes topically administering the composition to a mammal in need of such treatment. The present invention also provides for kits and syringe systems that include the adenosine deaminase inhibitor.
Owner:QLT USA INC
Medicinal composition for treating non-small cell lung cancer and application thereof
InactiveCN103948689AReduce dosageLow toxicityAntineoplastic agentsHeavy metal compound active ingredientsSalvia miltiorrhizaCarboplatin
The invention discloses a medicinal composition for treating non-small cell lung cancer. The medicinal composition comprises a target medicament, a chemotherapeutic medicament and a traditional Chinese medicament, wherein the target medicament is one or more of bortezomib, imatinib, gefitinib and sunitinib; the chemotherapeutic medicament is one or more of 5-fluorouracil, carboplatin, epirubicin, adriamycin and fludarabine; and the traditional Chinese medicament is one or more of salvia miltiorrhiza, astragalus membranaceus, sappanwood, Chinese pulsatilla root and portulaca oleracea. The medicinal composition has a remarkable synergetic treatment effect when being used for treating the non-small cell lung cancer, and can be used for remarkably strengthening the cancer-inhibition effect compared with treatment of a single medicament, so that the medicament dosage can be reduced, and the toxic and side effects of chemotherapeutic medicaments can be reduced.
Owner:NORTHWEST A & F UNIV
Methods of Conditioning Patients for T Cell Therapy
ActiveUS20160346326A1Improve the level ofPolypeptide with localisation/targeting motifOrganic active ingredientsRegulatory T cellT cell
The invention provides methods of increasing the efficacy of a T cell therapy in a patient in need thereof. The invention includes a method of conditioning a patient prior to a T cell therapy, wherein the conditioning involves administering a combination of cyclophosphamide and fludarabine.
Owner:UNITED STATES OF AMERICA +1
Novel therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
InactiveUS20050233987A1Improve toleranceImprove therapeutic potentialBiocideSugar derivativesDiseaseApoptosis
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
Phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer
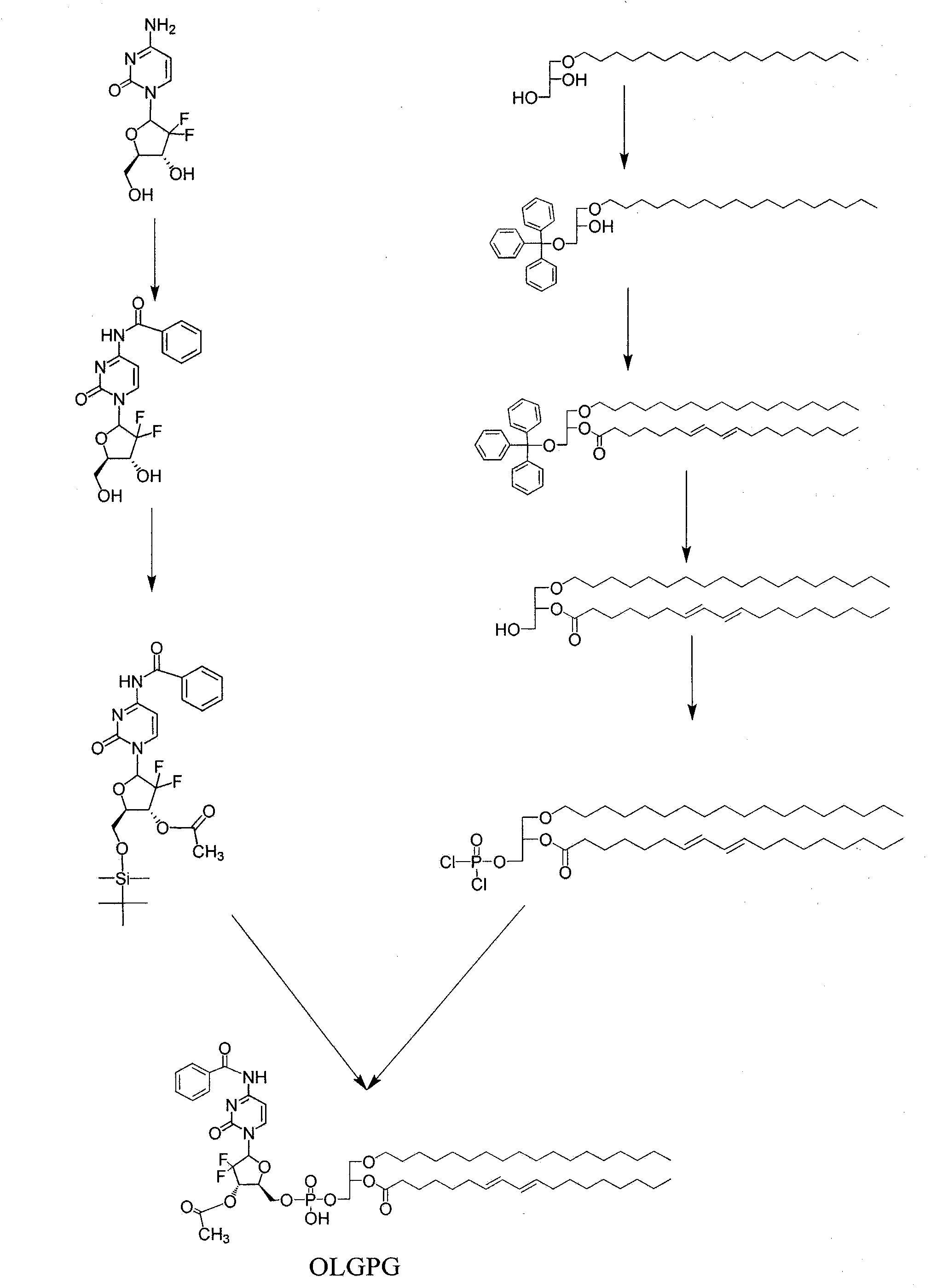
The invention discloses a phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer. The phosphoryl N-fatty acyl nucleoside analogue is characterized in that a nucleoside analogue is modified by a cyclophosphoryl group and then is connected to aliphatic chains having different numbers of carbon atoms. The phosphoryl N-fatty acyl nucleoside analogue can be used for convenient preparation of a nanometer transmission system and has obvious hepatocyte and tumor targeting. The nanometer transmission system comprises liposome, nonionic surfactant niosomes, nanoparticles, nano-emulsion and a self-assembled transmission system. The nucleoside analogue is selected from lamivudine, adenine arabinoside, cidofovir, gemcitabine, cytosine arabinoside, azacitidine and fludarabine. After intravenous administration, the nanometer transmission system of the phosphoryl N-fatty acyl nucleoside analogue has effects of targeting treatment on viral hepatitis and liver cancer.
Owner:ACADEMY OF MILITARY MEDICAL SCI
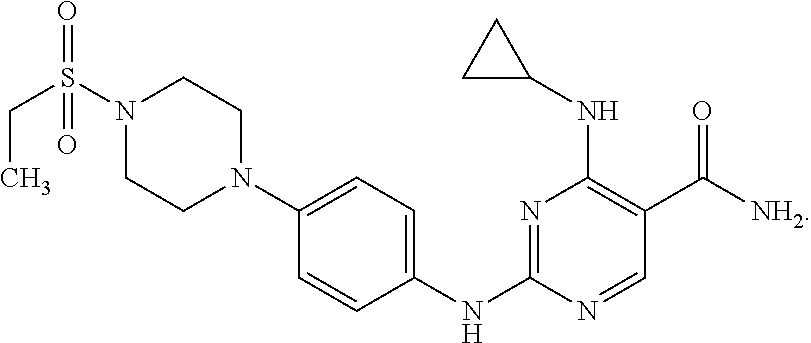
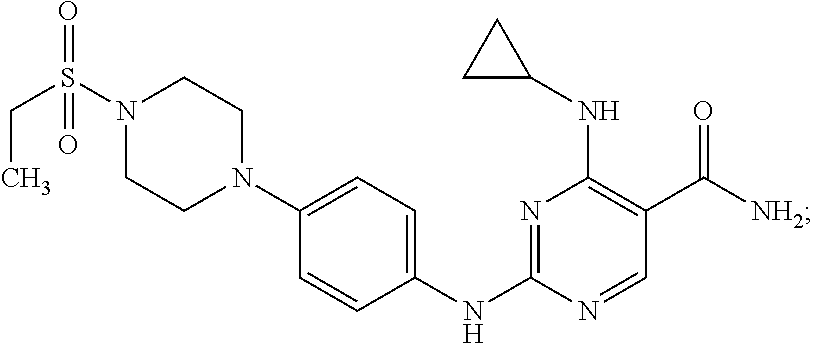
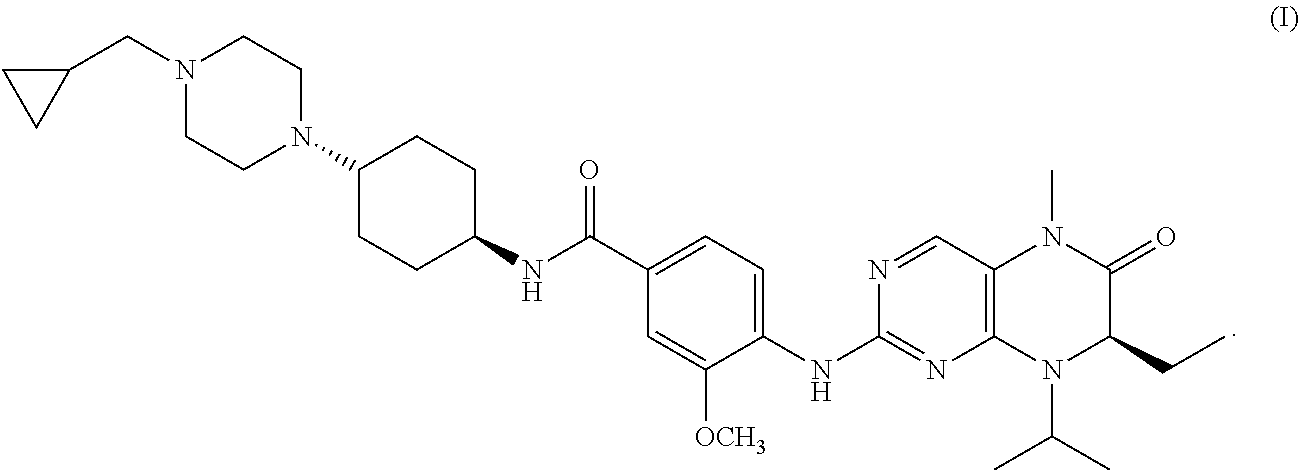
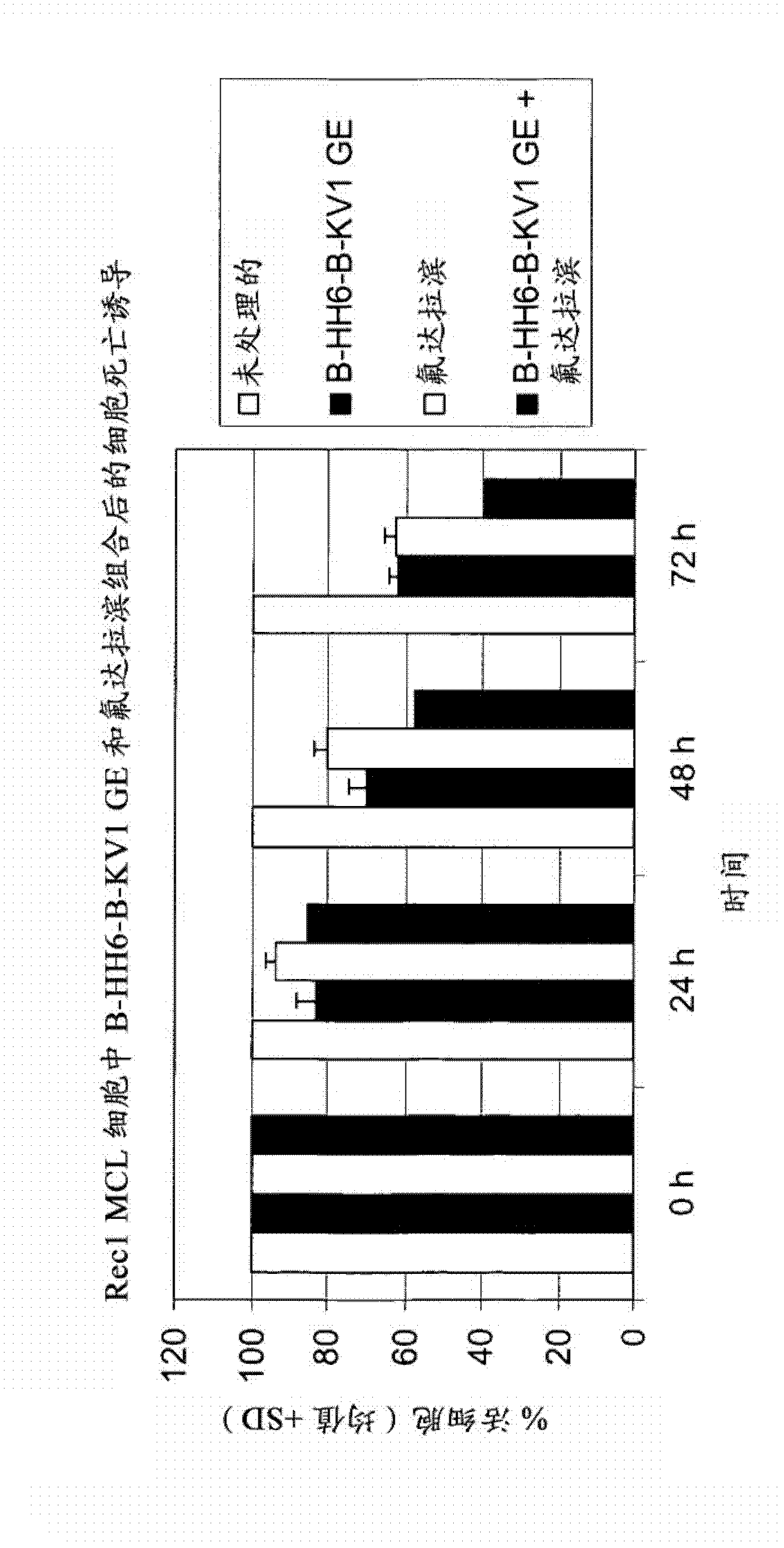
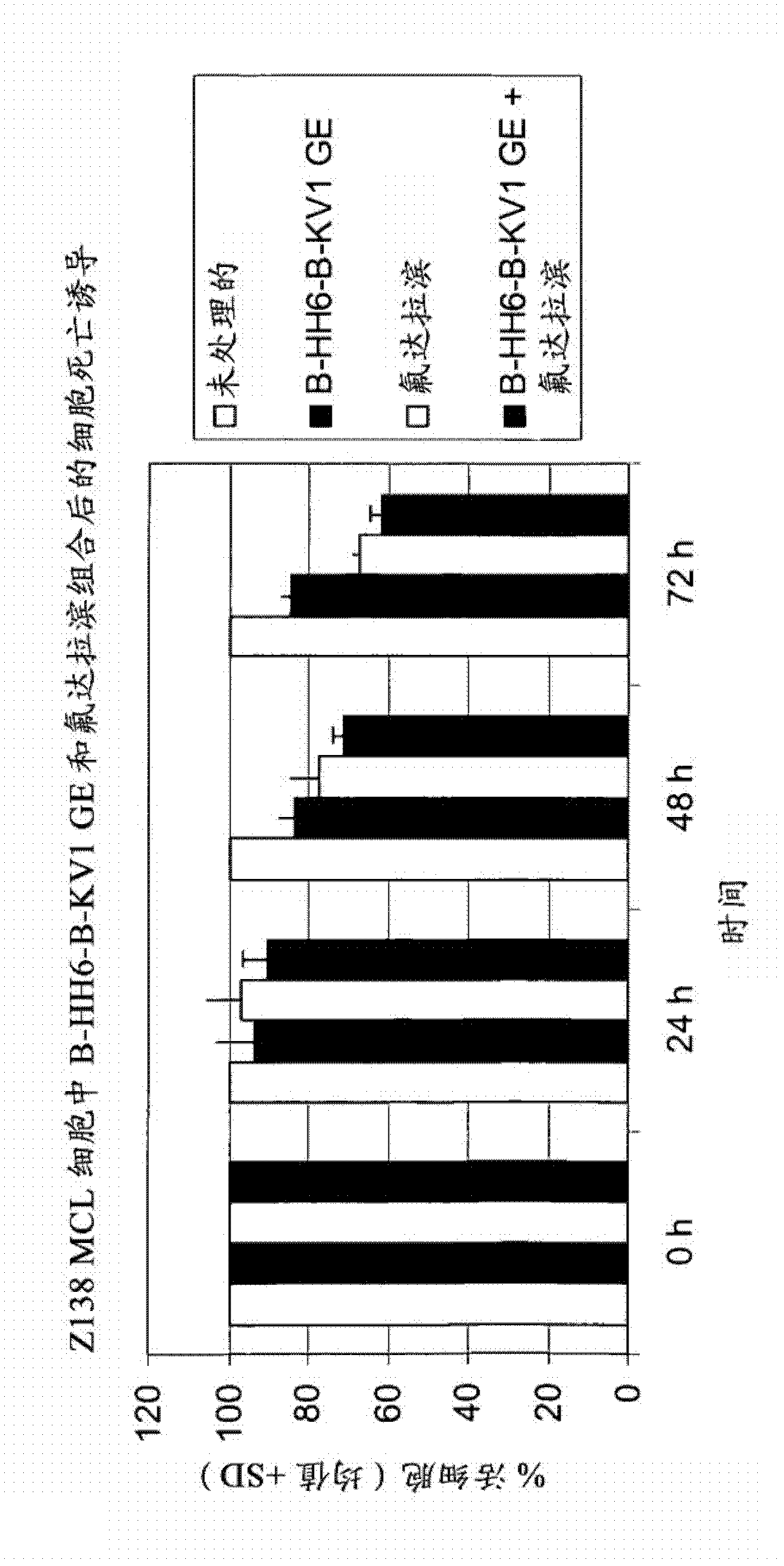
Combination therapy of 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide and fludarabine
InactiveUS20130237493A1Good effectGood treatment effectBiocideCarbohydrate active ingredientsMantle lymphomaCombination therapy
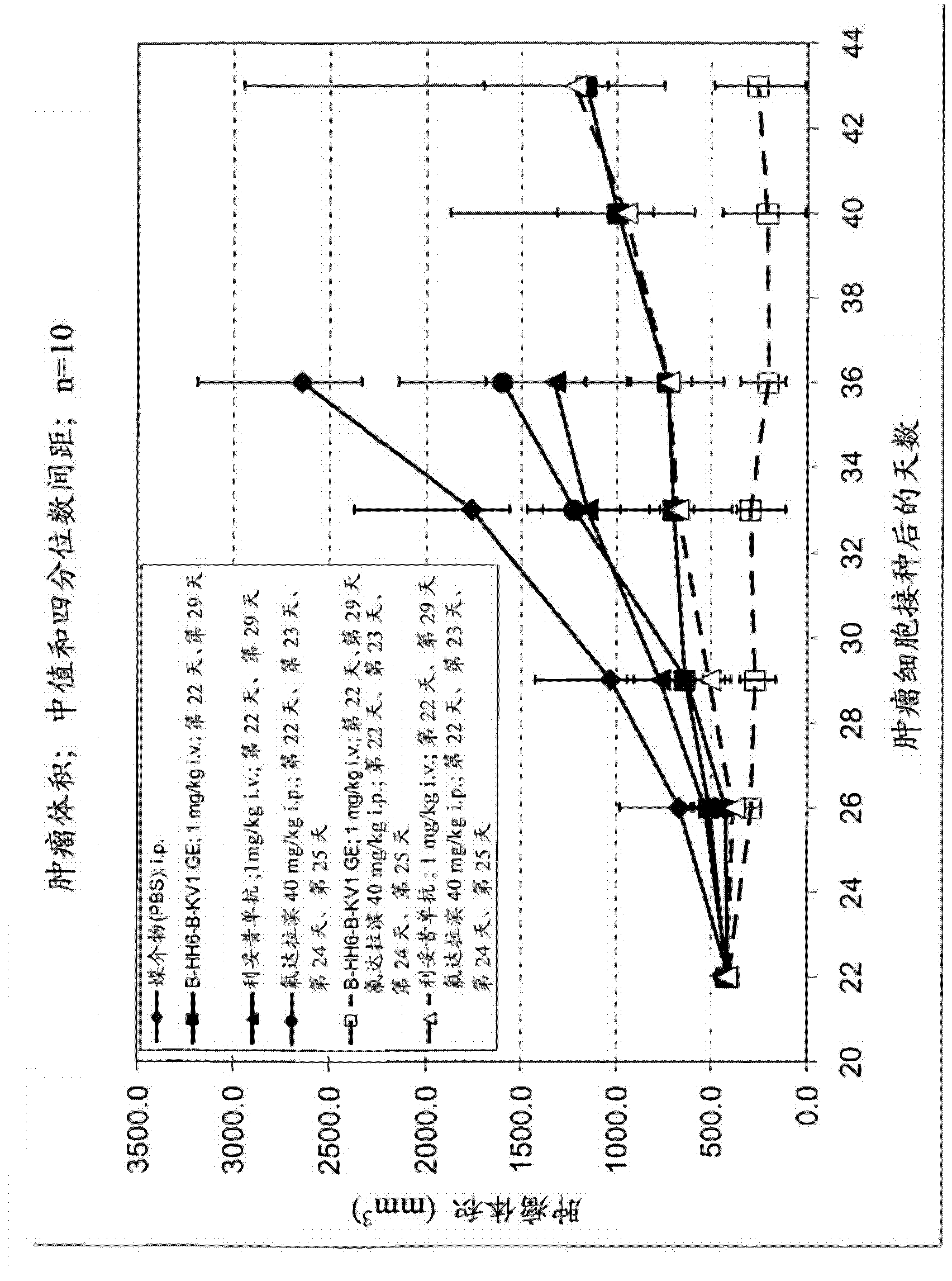
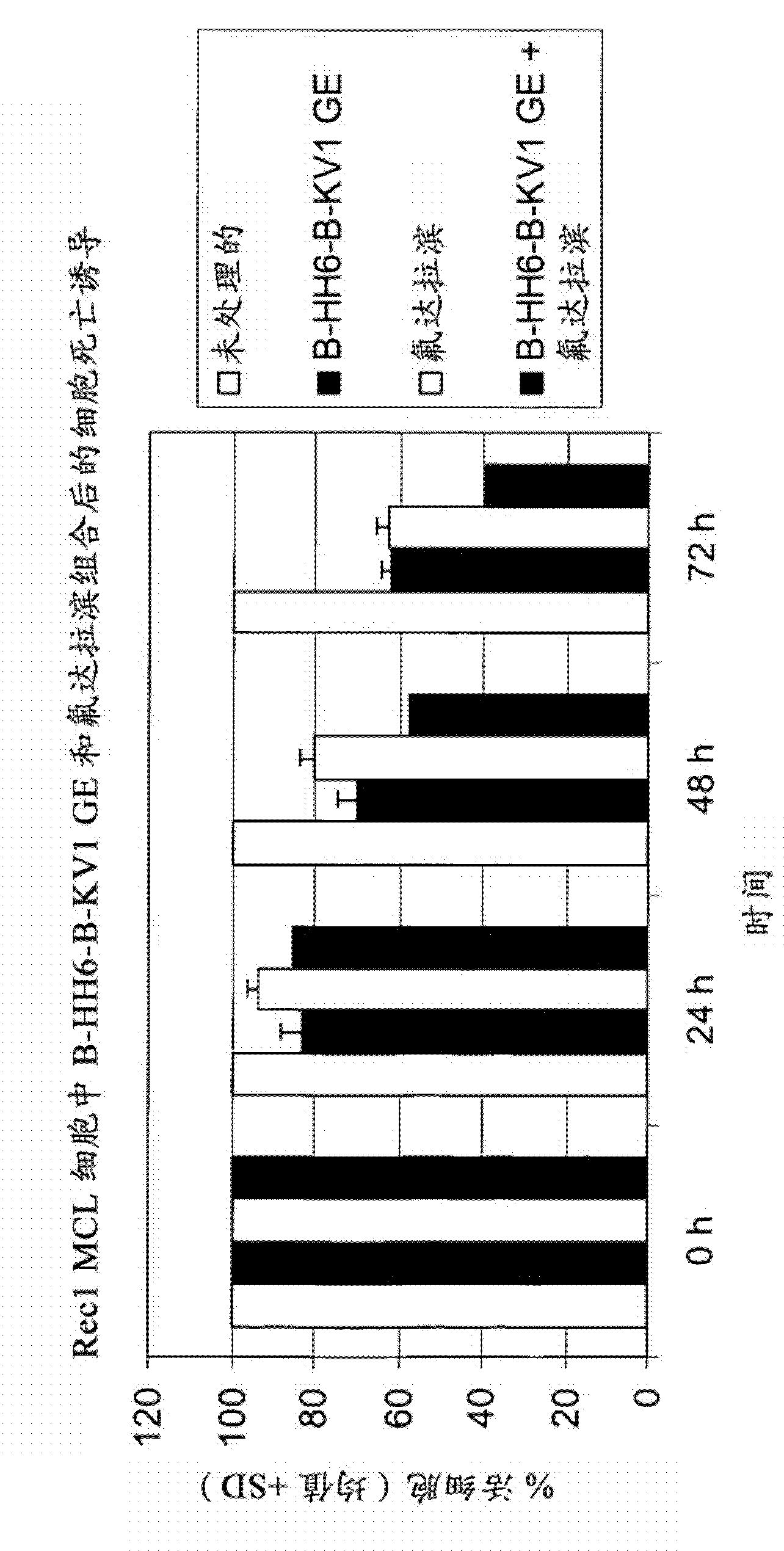
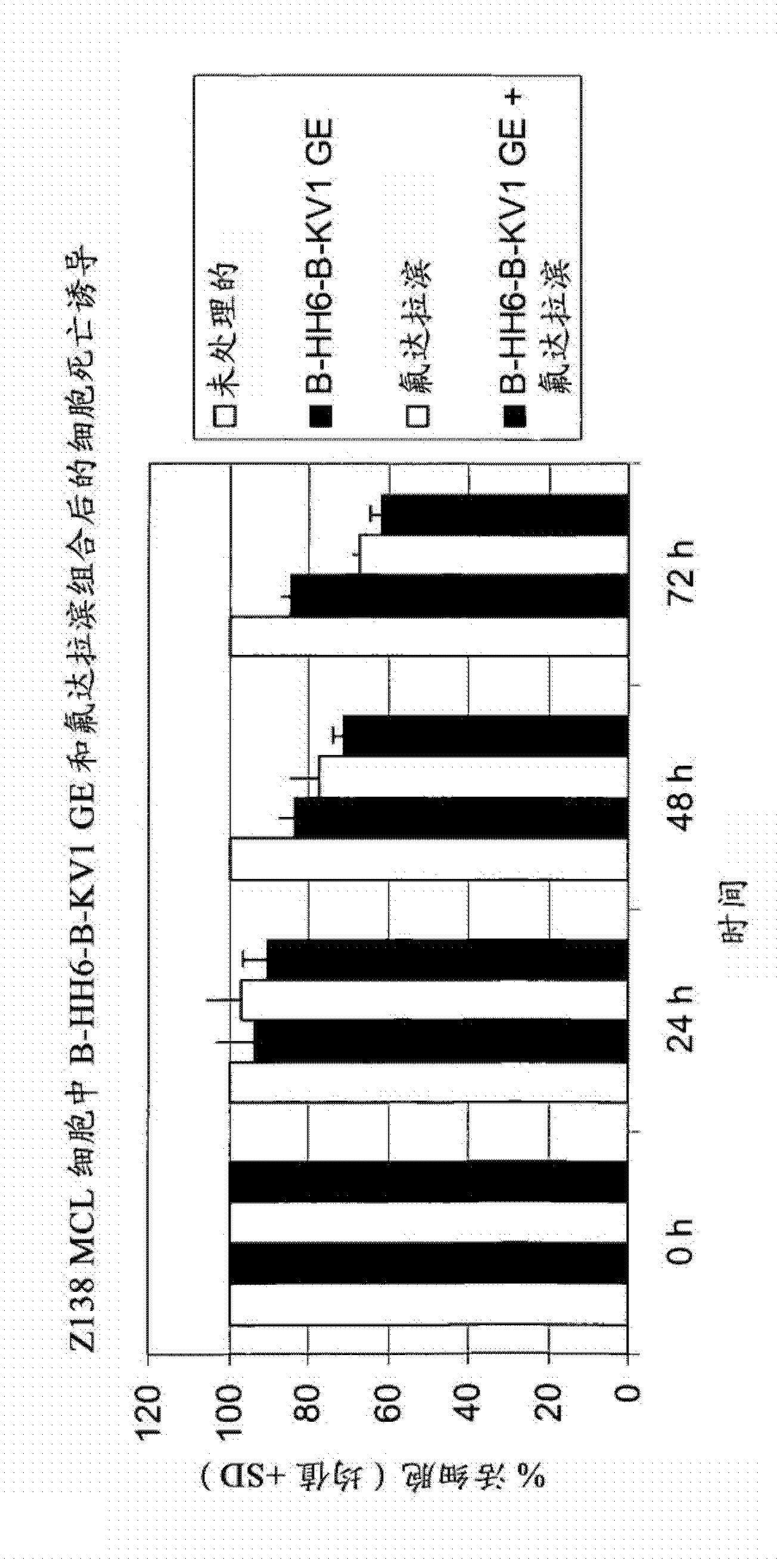
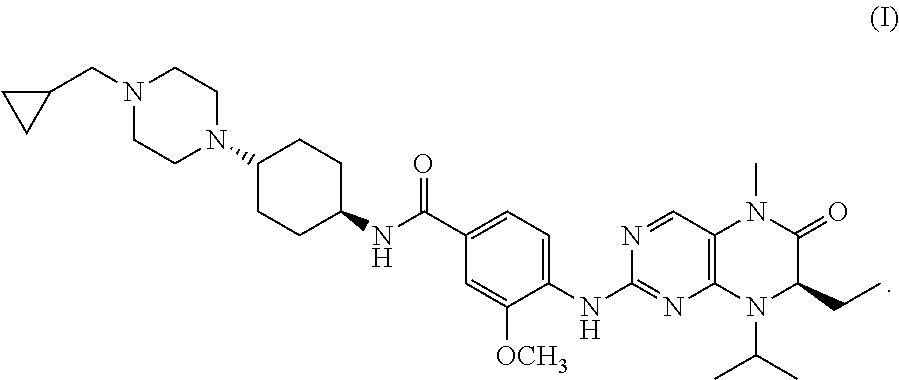
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide, or a pharmaceutically acceptable salt thereof, and fludarabine for the treatment of cell proliferative disorders, such as undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), including diffuse large B cell lymphoma (DLBCL); mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic Lymphoma (SLL) and multiple myeloma.
Owner:ALEXION PHARMA INC
Fludarabine sustained-release implantation agent for treating solid tumors
InactiveCN101219101AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to a solid tumors-curing fludarabine sustained-release implant, which is characterized in that the sustained-release implant contains effective anti-tumor dose of fludarabine, sustained-release auxiliary material and a certain amount of sustained-release regulator. Solid tumors consist of lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer and colorectal cancer. The sustained-release auxiliary materials are mainly one or mixture of copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate). When the sustained-release auxiliary materials are degrading and absorbed, fludarabine is slowly released into tumor part, thus while reducing toxic reaction of the whole body, the sustained-release auxiliary materials can sustain concentration of effective drug at the tumor part. Placing the anti-tumor sustained-release implant at the tumor part can not only reduce toxic reaction of the whole body of fludarabine, but also improve drug concentration at the tumor part and enhance treatment effects of nonspecific treatments, such as chemotherapeutics, radiotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Antimetabolites and potentiating agent thereof carried by anticancer sustained-release injection
InactiveCN101297970ASolution deliveryPharmaceutical non-active ingredientsSodium carboxymethylcelluloseCyclophosphamide/fludarabine
The invention disclose an anticancer slow-release injection of sync-loaded anti-metabolites and synergists thereof, which comprises slow-release microspheres and menstruum, wherein, the slow-release microspheres comprise anticancer active ingredient and slow-release auxiliary material, and the menstruum is a special menstruum containing a suspending agent. The anticancer active ingredient is an anti-metabolite selected from Zalcitabine, emtricitabine, Galocitabine, ibacitabine, ancitabine, Decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, Gemcitabine, Fludarabine or Cladribine and / or the synergist of the anti-metabolite selected from hormone anticancerogen and / or platinum compounds; the slow-release auxiliary material is selected from one or the combination of bi-fatty acids and sebacic acid copolymer, poly-(erucic acid dimer-sebacic acid), poly-( fumaric acid-sebacic acid), polifeprosan, polylactic acid copolymer and EVAc; the suspending agent is selected from carboxymethyl cellulose, etc. and the viscosity of the suspending agent ranges from 80cp to 3000cp (at the temperature of 20 DEG C to 30 DEG C). The slow-release microspheres can be produced to be a slow-release implant to be injected or placed in a tumour or the periphery of the tumour, and are independently used or used with non-operative treatment such as radiotherapy, chemotherapy or microwave, etc.
Owner:SHANDONG LANJIN PHARMA +1
Therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
The present disclosure relates to a method of treatment of a human patient suffering from a B-cell lymphoproliferative disorders such as B-cell chronic lymphocytic leukemia (B-CLL), splenic marginal zone lymphoma (SMZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), lymphoplasmacytic lymphoma (LPL), and Waldenström syndrome (WS), by the administration of a therapeutically effective amount of 5-aminoimidazole-4-carboxamide riboside (acadesine) or its precursors (eg. its mono-, di- and tri-5′-phosphates). This makes acadesine and its bioprecursors (eg. its mono-, di- and tri-5′-phosphates) useful as therapeutic agents for B-cell lymphoproliferative disorders in humans. The surprising feature that T cells are virtually not affected means that the side effect (immunosuppression) is minor, what represents a therapeutical advantage of acadesine over cladribine, fludarabine and other nucleosides known in the art.
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
Methods of conditioning patients for t cell therapy
ActiveUS20170368101A1Improve the level ofPolypeptide with localisation/targeting motifOrganic active ingredientsSurgeryCell therapy
The invention provides methods of increasing the efficacy of a T cell therapy in a patient in need thereof. The invention includes a method of conditioning a patient prior to a T cell therapy, wherein the conditioning involves administering a combination of cyclophosphamide and fludarabine.
Owner:KITE PHARMA INC +1
Anti-cancer drug slow release injection containing gemcitabine
The invention relates to an anti-cancer drug slow release injection containing gemcitabine, which comprises slow release microspheres and a menstruum, wherein the microsphere comprises anti-cancer active ingredients and slow release auxiliary materials, and the menstruum is a special menstruum containing a suspending agent; the anti-cancer active ingredients are gemcitabine, or antimetabolites selected from zalcitabine, emtricitabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazole gemcitabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite potentiating agents selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogue and / or DNA repair enzyme inhibitor; the slow release auxiliary materials are polifeprosan, dienoic fatty acid, decanedioic acid copolymer, polylactic acid copolymer, EVAc and the like; the suspending agent has a viscosity of 100cp-3000cp (20-30 DEG C), and is selected from sodium carboxymethylcellulose and the like. The slow release microspheres can be prepared into slow release implants. When injected or placed into tumors or at peripheries of tumors, the slow release microspheres can enhance the treatment effect of non-operative treatment methods, such as radiotherapy, chemotherapy, etc.
Owner:SHANDONG LANJIN PHARMA +1
Use of CD23 Antibodies to Treat Malignancies in Patients with Poor Prognosis
InactiveUS20090252725A1Induce apoptosisTherapeutically effectiveAntibody ingredientsTissue cultureMammalZAP70
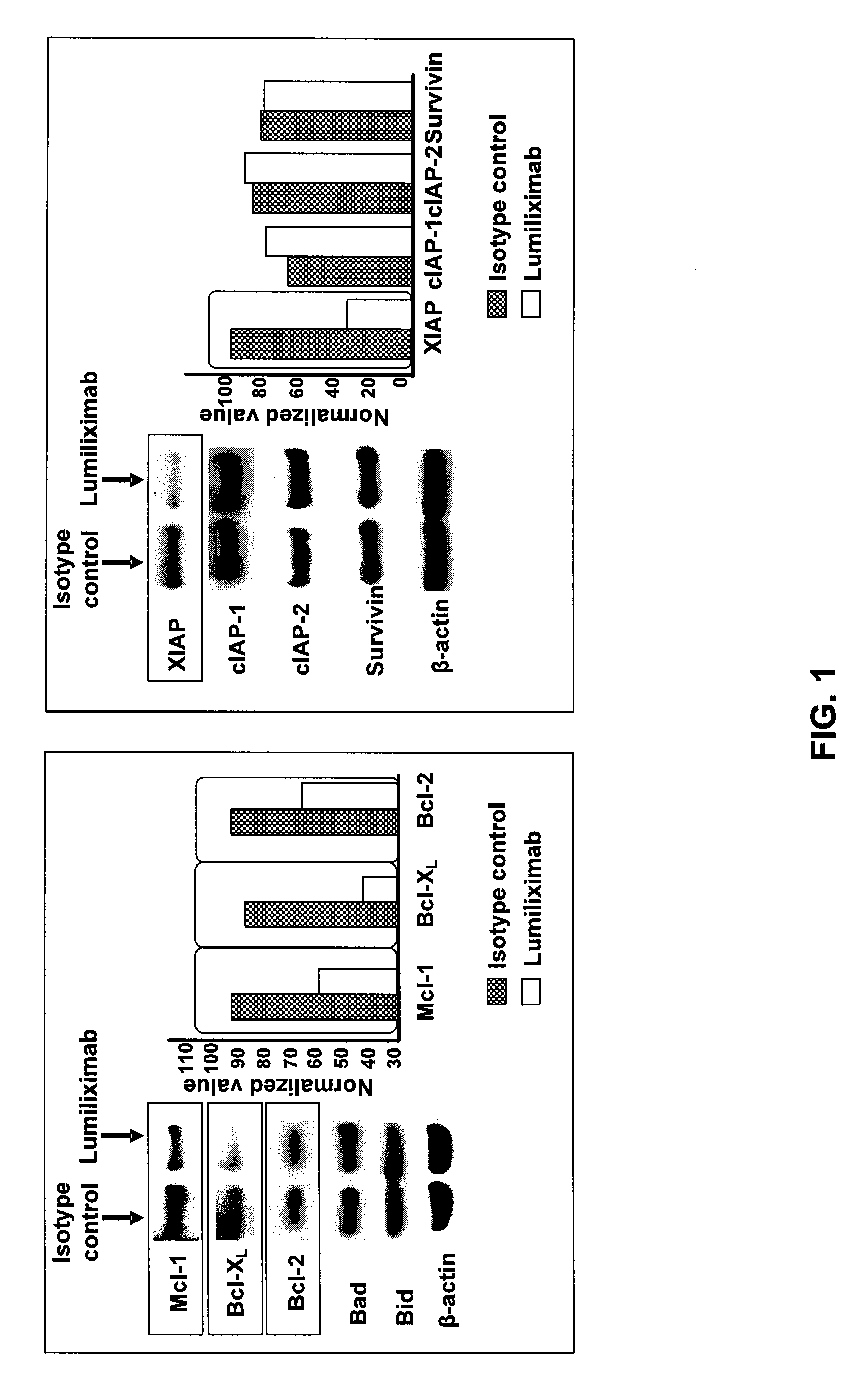
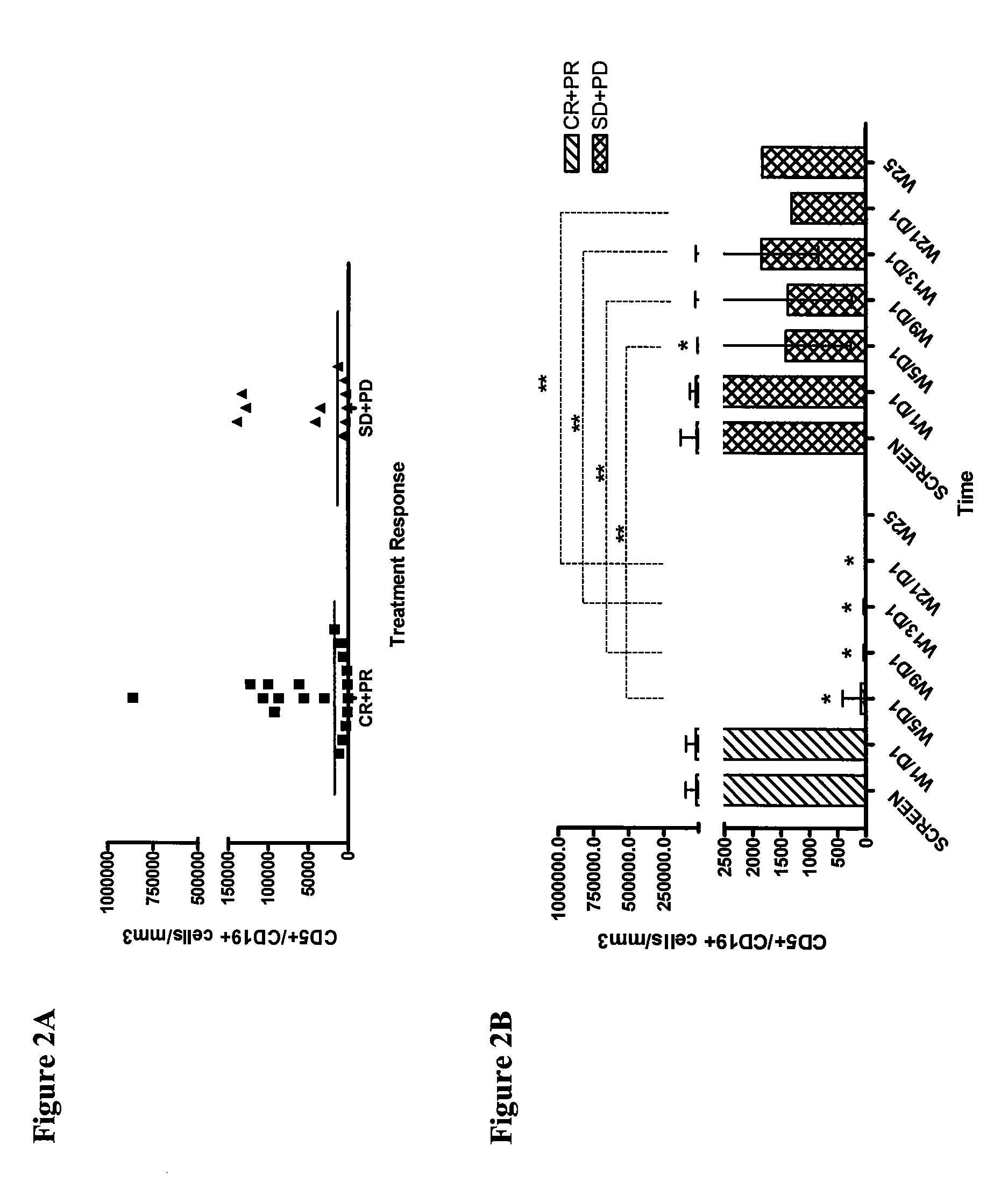
The invention relates to methods of treating B-cell chronic lymphocytic leukemia, and other CD23+ malignancies, in patients with poor prognostic markers. The method comprises administration of an CD23 antibody, including for example, lumiliximab to a mammal that over expresses a poor prognostic marker. The method can also comprise administration of lumiliximab in combination with fludarabine, cyclophosphamide and rituximab. Patients with poor prognostic markers include, for example, patients that overexpress ZAP70, CD38, β2-microglobulin and / or soluble CD23.
Owner:BIOGEN IDEC MA INC
Phospholipase A2 sensitive glycerin skeleton anti-tumor prodrug and high-dispersing preparation thereof
InactiveCN104208079AEfficient anti-tumor effectOrganic active ingredientsSolution deliveryCytarabineGlycerol
The invention discloses a phospholipase A2 sensitive glycerin skeleton anti-tumor prodrug of which a molecular structure is a glycerin skeleton including a long-chain alkyl ether at a 1st position, a conjugated linoleoyl at a 2nd position and a phosphoryl nucleoside at a 3rd position. Because that a tumor tissue can highly express phospholipase A2, the glycerin skeleton anti-tumor prodrug has a tumor environmental specificity and can release a plurality of components which have activities on tumor cells at the position of the tumor. The components work in combination so that a high-efficient anti-tumor effect is achieved. An active compound of a nucleoside-type anti-tumor drug is selected from cytosine arabinoside, gemcitabine, capecitabine, fludarabine and derivatives thereof. The glycerin skeleton anti-tumor prodrug can be prepared into following high-dispersing preparations: a liposome, a nonionic surfactant vesicle, nano particles, a nano emulsion or a self-assembling transmission system.
Owner:ACADEMY OF MILITARY MEDICAL SCI
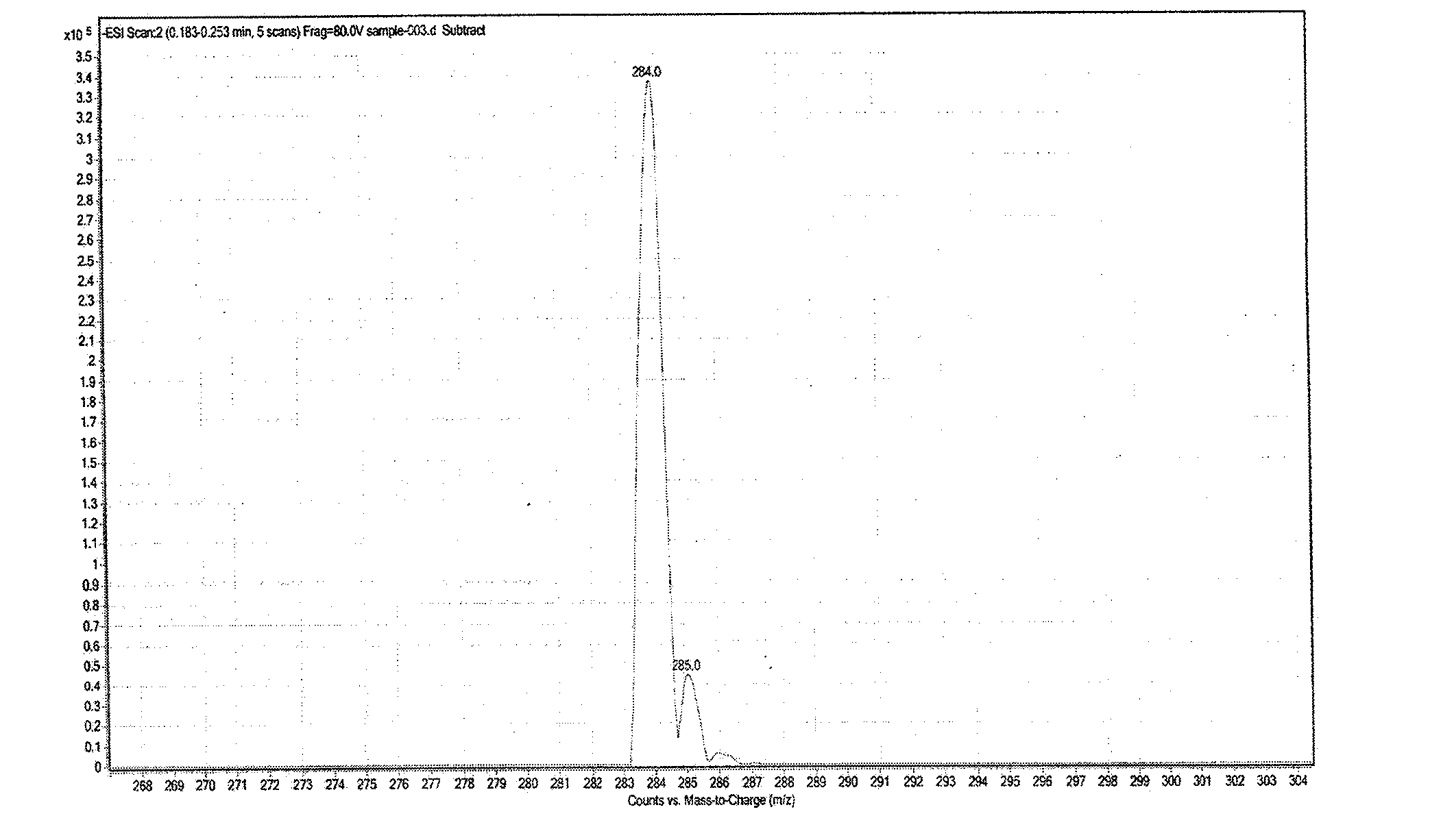
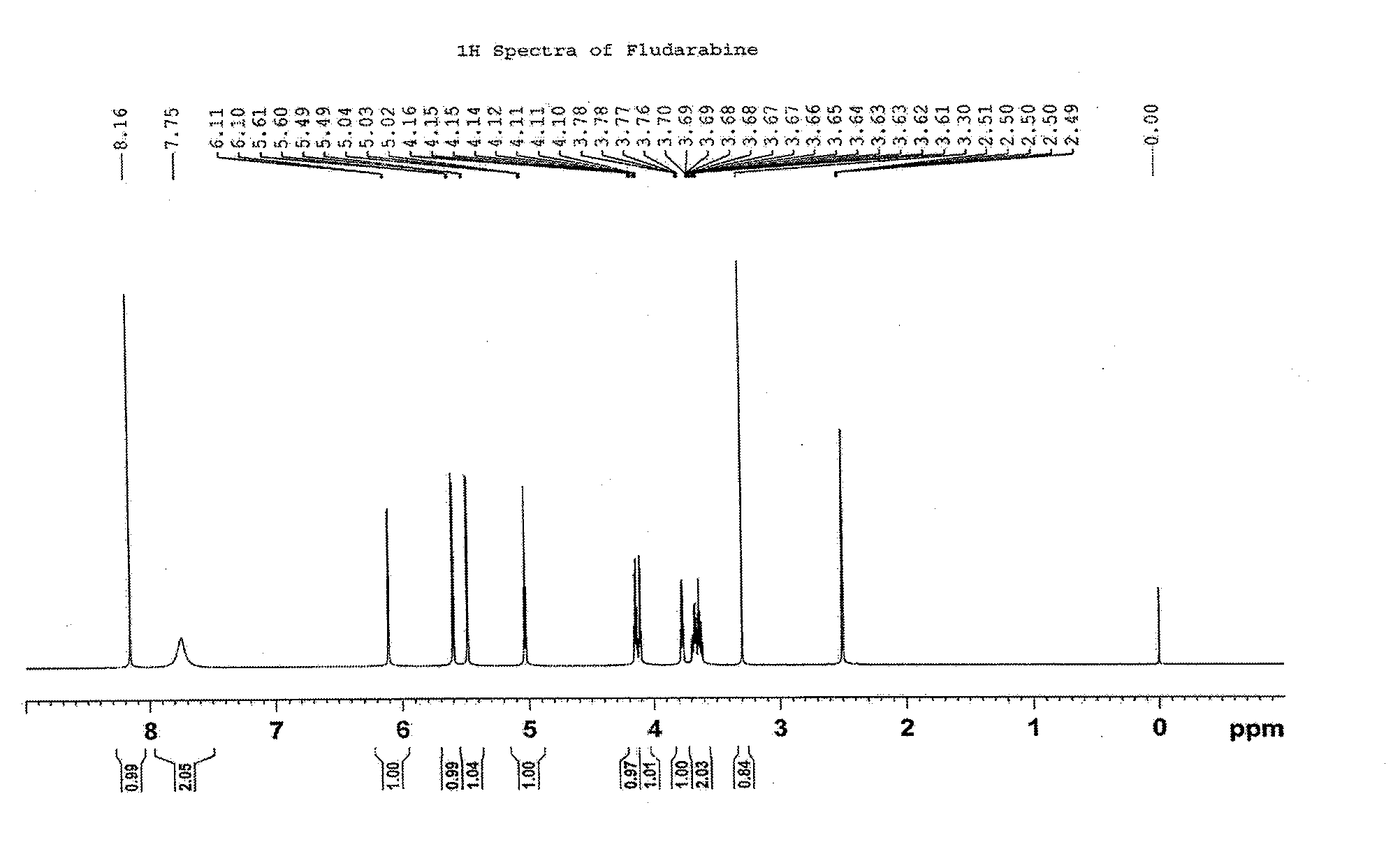
Synthesis method of fludarabine
InactiveCN102911230AReduce pollutionEasy to purifySugar derivativesSugar derivatives preparationAcetic acidActivated carbon
The invention discloses a novel method for synthesizing fludarabine, which comprises the following steps: by using 2-fluoro-9-beta-D-(2',3',5'-tri-alkoxyarabinofuranosyl)adenine as a raw material, a sodium hydroxide and ammonia water mixed solution as a reaction reagent and a water and 2-methyltetrahydrofuran mixed solution as a solvent, carrying out reaction at 0-5 DEG C for 1-3 hours, neutralizing with glacial acetic acid, carrying out vacuum filtration, recrystallizing the obtained crude solid with water, and decolorizing with activated carbon to obtain the pure fludarabine. The preparation method disclosed by the invention uses the mixed solution of water and green solvent 2-methyltetrahydrofuran as the solvent, and thus, is environment-friendly. Besides, compared with the existing method, the synthesis method has the advantages of short reaction time, cheap raw material and the like, and is safe and simple to operate; and the product is easy to purify. The invention is suitable for industrial production.
Owner:HUAIHAI INST OF TECH
Gemcitabine-containing anti-cancer medicine sustained-release injection
The invention relates to cancer therapy drug sustained-release injection containing gemcitabine. The injection comprises sustained-release microspheres and dissolvent, wherein, the sustained-release microspheres comprise active ingredients for cancer therapy and sustained-release auxiliary materials, and the dissolvent is special dissolvent containing suspending agent. The active ingredients for cancer therapy comprise antimetabolite of gemcitabine or antimetabolite selected from zalcitabine, emtritabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite synergist selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogs and / or DNA repair enzyme inhibitor; the sustained-release auxiliary materials comprise polifeprosan, bi-fatty acid, decanedioic copolymer, polylactic copolymer and EVAc; the viscocity of the suspending agent is 100cp to 3000cp (at 20 to 30 DEG C) and the suspending agent is selected from sodium carboxymethyl cellulose. The sustained-release microspheres can also be produced into sustained-release implant, and by injecting or positioning the sustained release agent in tumor or tumor margin, the efficacy of non-operative treatment such as radiation treatment and chemical treatment can be enhanced.
Owner:SHANDONG LANJIN PHARMA +1
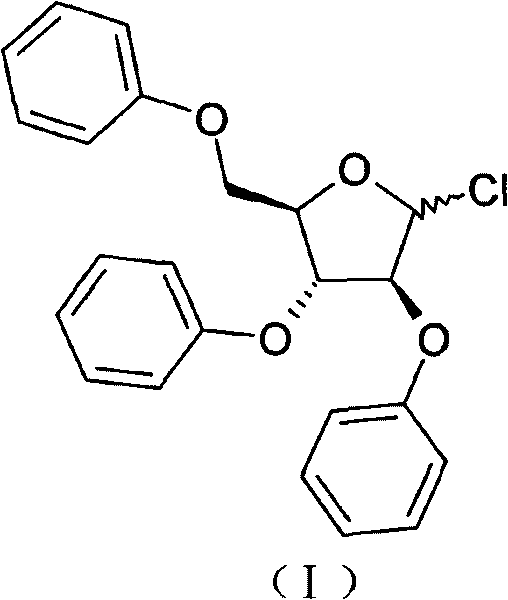
Method for preparing 2,3,5-tri-O-tribenzyl-1-chloro-D-arabinofuranose
The invention relates to 2,3,5-tri-O-tribenzyl-1-chloro-D-arabinofuranose, which is used for producing important intermediates of anti-cancer drugs, such as nelarabine, fludarabine, ancitabine and the like. The invention relates to a method for preparing the 2,3,5-tri-O-tribenzyl-1-chloro-D-arabinofuranose. The method can acquire a solid-state target product which is convenient to store, transport and use.
Owner:邓俐丽
Synthetic method of 2-fluoroadenosine
InactiveCN103333215ARaw materials are cheap and easy to getMild reaction conditionsSugar derivativesSugar derivatives preparationMolecular sieveOrganic solvent
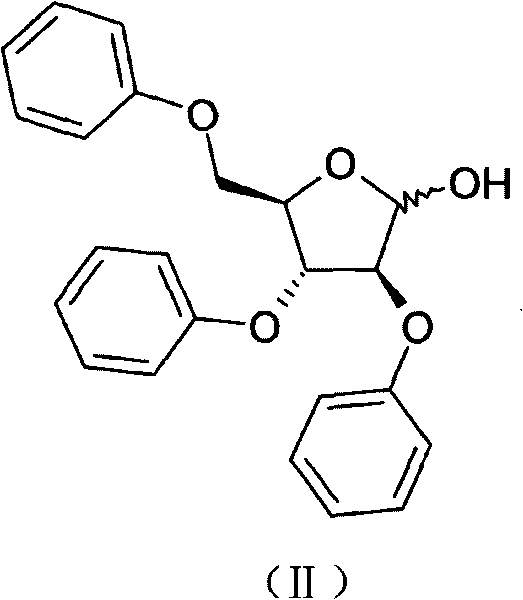
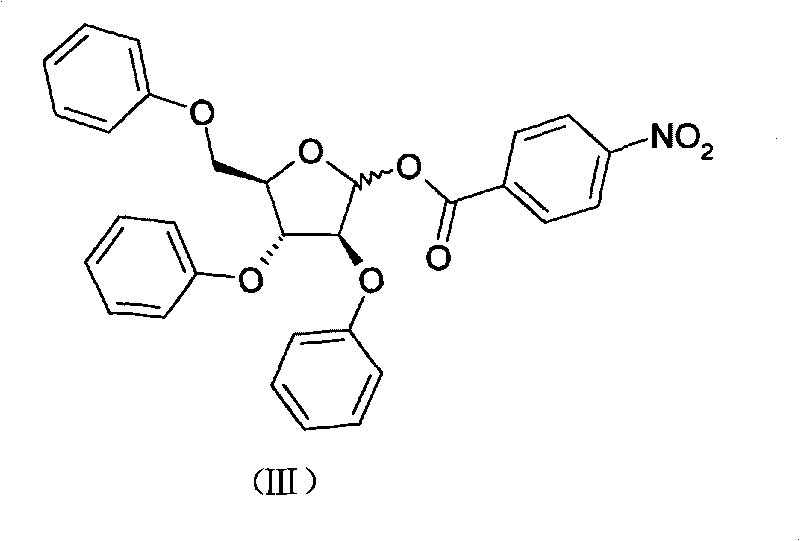
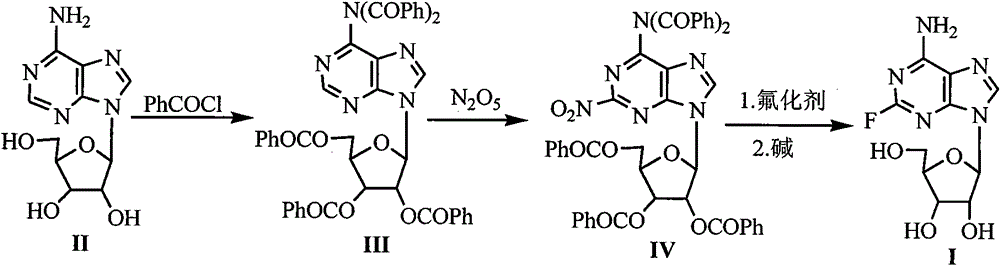
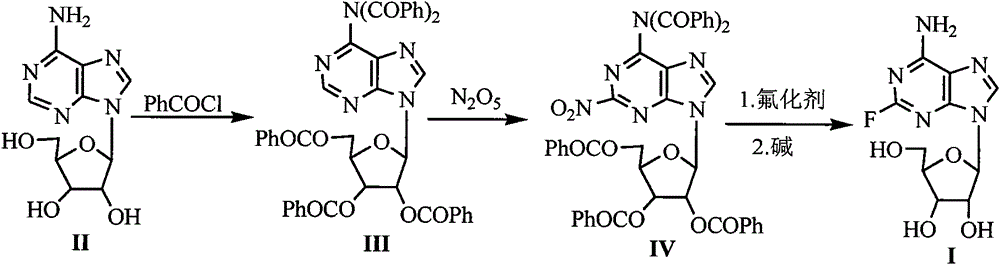
The invention discloses a synthetic method of 2-fluoroadenosine which is an important intermediate of fludarabine. The synthetic method comprises following steps: (1) a compound represented by formula (II) is reacted with benzoyl chloride in pyridine, and a compound represented by formula (III) is obtained; (2) the compound represented by formula (III) and N2O5 which is a cheap nitrating agent are subjected to nitration reaction in the presence of home-made HZSM-5 molecular sieve catalyst, and a compound represented by formula (IV) is obtained; (3) the compound represented by formula (IV) is reacted with a cheap fluorinating agent in an organic solvent in the presence of a catalyst, 2-fluoroadenosine with a protective group is obtained, and then the protective group is removed in a mixed solvent under alkaline conditions, and a compound represented by formula (I) is obtained. The raw materials of the synthetic method of 2-fluoroadenosine of the invention are cheap and easily available; reaction conditions are mild; operational procedures are simple; synthetic efficiency is high; the method is environment-friendly, and is suitable for industrialized production.
Owner:HUAIHAI INST OF TECH
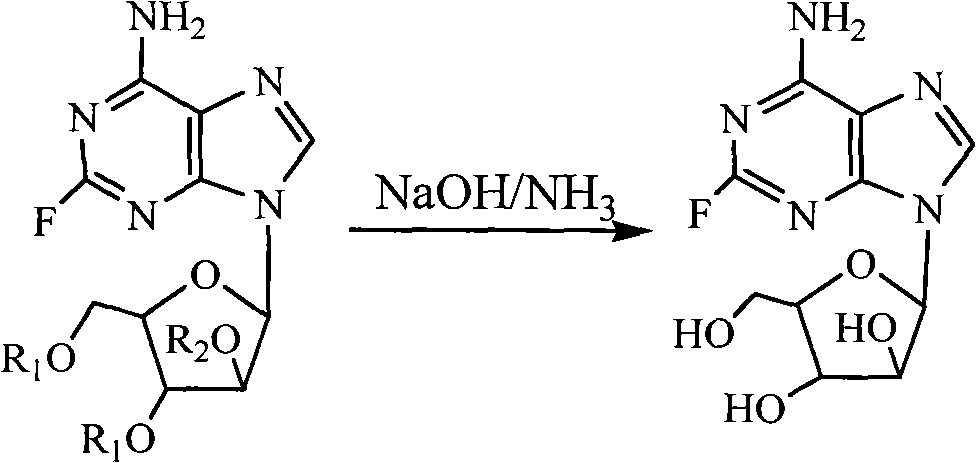
Method for preparing fludarabine
ActiveCN101735296ACreate pollutionLow costSugar derivativesSugar derivatives preparationSolventAmmonia
The invention relates to a method for preparing fludarabine, which takes 2-fluoro-9-beta-D-(2',3',5'-tri-O-acetyl arabinofuranose) adenine as a raw material, saturated aliphatic alcohols as a solvent, and ammonia water as a reagent, and comprises the following steps: reacting for 24 to 48 hours at the temperature of between -5 and 15 DEG C; filtering the mixture to obtain a crude product; and finally, recrystallizing the crude product to obtain the fludarabine. The method for preparing the fludarabine takes the saturated aliphatic alcohols as the solvent and does not use a chloric solvent so as not to pollute environment. In addition, compared with the prior art, the preparation method has the advantages of short reaction time, low cost of the raw material, high product purity, simple operation and the like so as to be suitable for industrialized production.
Owner:TIANJIN WEIJIE TECH
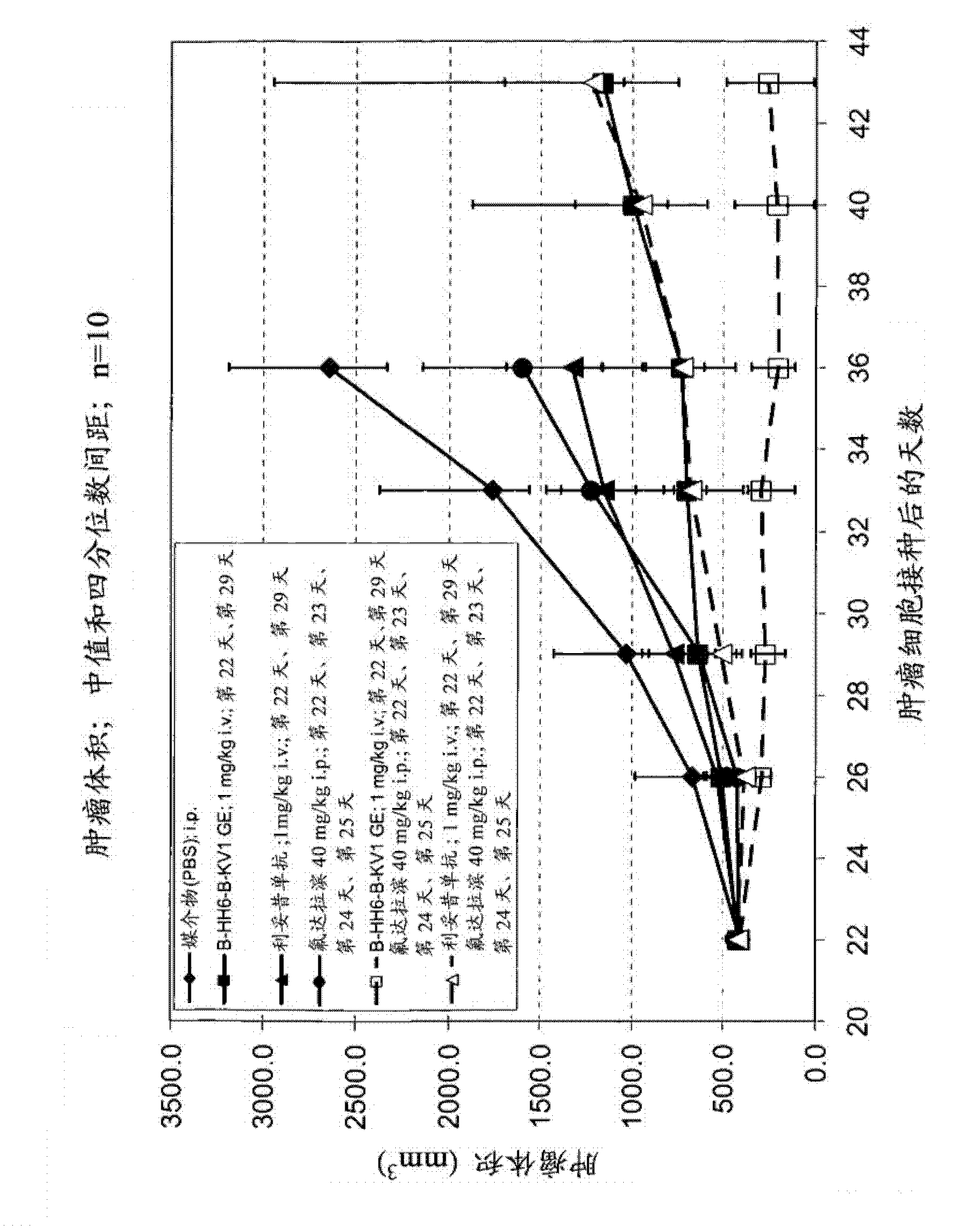
Combination therapy with volasertib
Disclosed is the use of Volasertib or a salt or a hydrate thereof for treating patients suffering from acute myeloid leukemia (AML) comprising administering a high dose of Volasertib in combination with fludarabine, cytarabine and Granulocyte colony-stimulating factor (GCSF) or in combination with fludarabine, cytarabine, GCSF and a daunorubicin citrate liposome injection.
Owner:BOEHRINGER INGELHEIM INT GMBH
Combination therapy of an afucosylated CD20 antibody with fludarabine and/or mitoxantrone
InactiveCN102470172AImmunoglobulins against cell receptors/antigens/surface-determinantsCarbohydrate active ingredientsFucosylationMitoxantrone
The present invention is directed to the combination therapy of an afucosylated anti-CD20 antibody with fludarabine and / or mitoxantrone for the treatment of cancer, especially to the combination therapy of CD20 expressing cancers with an afucosylated humanized B-Ly1 antibody with fludarabine and / or mitoxantrone.
Owner:ROCHE GLYCART AG
Fludarabine phosphate preparation method
InactiveCN105859812AResidue reductionMild reaction conditionsSugar derivativesSugar derivatives preparationOrganic solventTriethylamine phosphate
The invention discloses a fludarabine phosphate preparation method. The method comprises the specific steps that fludarabine and triethyl phosphate are added into a reaction container, the reaction container is placed into a subzero 6 DEG C low-temperature reaction bath, phosphorus oxychloride is added on the stirring condition, water and dichloromethane are added into the reaction container after the reaction is performed for 12 h, standing is performed, then, extraction is performed to obtain a water phase and an organic phase, the pH value of the water phase is adjusted to 2-3, recrystallization is performed to obtain white focculus, and filtering and vacuum drying are performed to obtain a target product of fludarabine phosphate with the purity being 99.95%. The method has the advantages that reaction conditions are mild, operation is easy, the product is easy to separate and purify, the yield is high, the product is environmentally friendly, high in purity, small in organic solvent residual quantity and capable of meeting the medicinal standard, and the method is suitable for industrial production.
Owner:HENAN NORMAL UNIV
Universal cart/tcrt cells with chemotherapeutic drug resistance and construction method thereof
ActiveCN107746831BPreserve targetingAvoid exclusionImmunoglobulin superfamilyTransferasesAntigenAntigen receptors
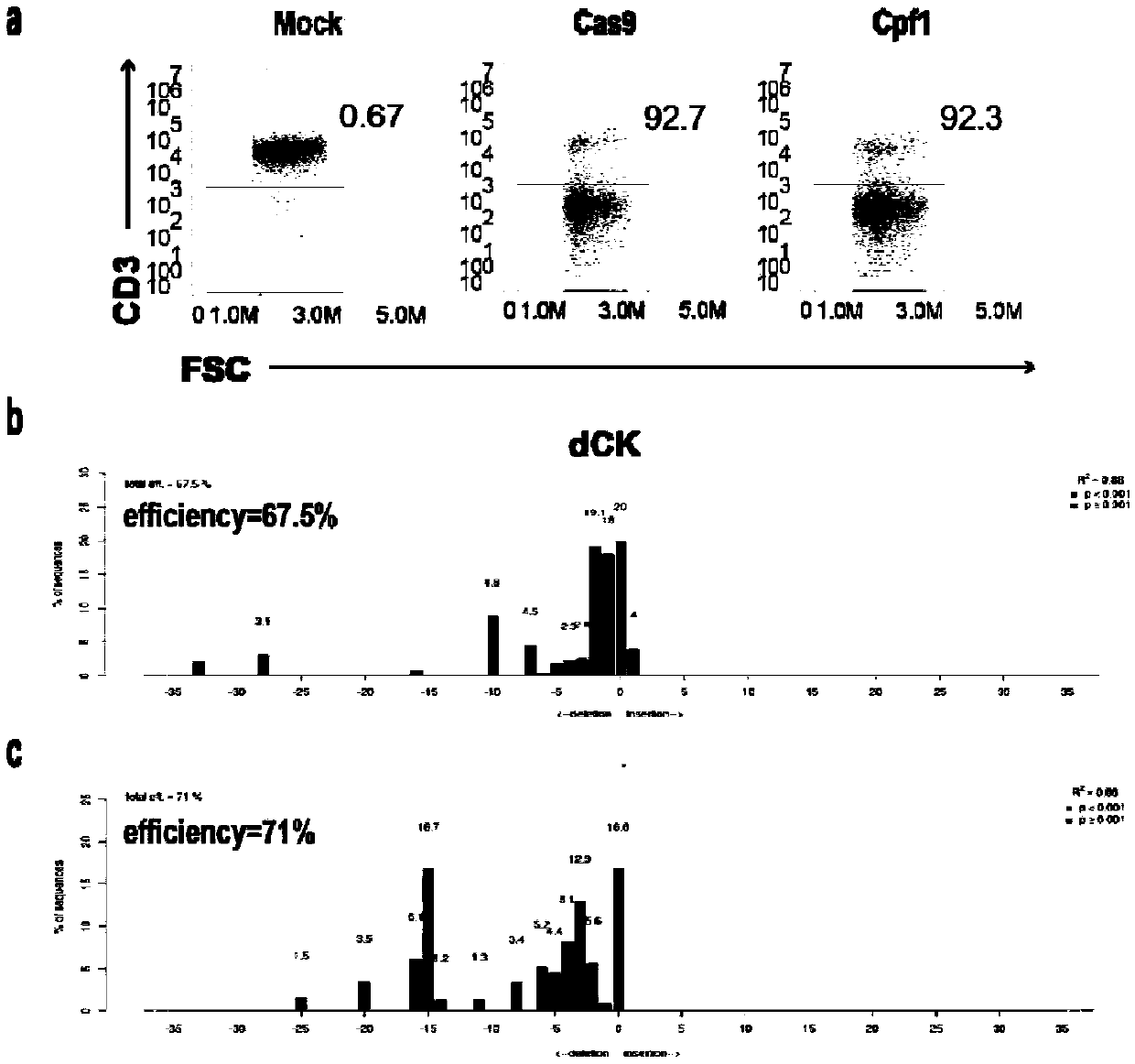
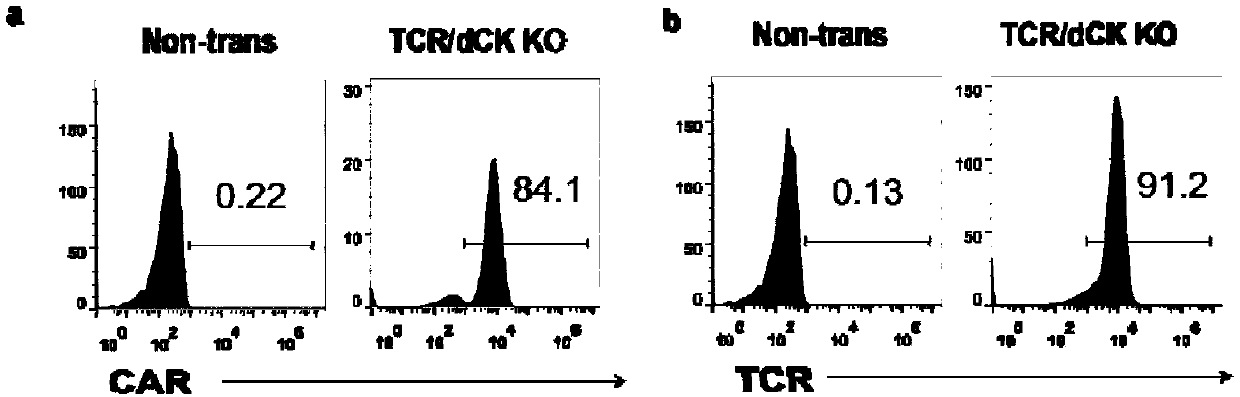
The invention belongs to the fields of genetic engineering and synthetic biology, and particularly relates to a universal CART / TCRT cell with chemotherapeutic drug resistance and a construction methodof the universal CART / TCRT cell. The universal CART / TCRT cell is an allogeneic T cell with chimeric antigen receptors and T cell receptors, wherein the alpha and beta chains of the T cell receptors and deoxycytidine kinase molecules are knocked out; and the knocking-out technology for the alpha and beta chains of the T cell receptors and the deoxycytidine kinase molecules is a CRISPR technology;for the universal CART / TCRT cell, the targeting property of the tumor specific antigen is reserved, the problems of GvHD and rejection are also eliminated, meanwhile, the sensitivity for the chemotherapeutic medicines Fludarabine and Clofarabine is weakened, and the universal CART / TCRT cell can be used as a universal simple, low-cost and high-activity CART / TCRT cell preparation.
Owner:NANJING BIOHENG BIOTECH CO LTD
Mutations in SF3B1 and Chronic Lymphocytic Leukemia
InactiveUS20130164746A1Reduced survivalImprove the immunityMicrobiological testing/measurementAntineoplastic agentsCyclophosphamide/fludarabineMutation
The disclosure provides methods of prognosing a subject with CLL and determining the response of the subject to treatment with fludarabine by determining the presence or absence of mutations within the SF3B1 gene.
Owner:ROSSI DAVIDE +2
Combination therapy of an afucosylated CD20 antibody with fludarabine and/or mitoxantrone
InactiveCN102470172BImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFucosylationMitoxantrone
The present invention relates to a combination therapy of afucosylated anti-CD20 antibody and fludarabine and / or mitoxantrone for the treatment of cancer, in particular to the use of afucosylated humanized B-Ly1 antibody Combination therapy with fludarabine and / or mitoxantrone for the treatment of CD20-expressing cancers.
Owner:ROCHE GLYCART AG
Combination therapy with volasertib
Disclosed is the use of Volasertib or a salt or a hydrate thereof for treating patients suffering from acute myeloid leukemia (AML) comprising administering a high dose of Volasertib in combination with fludarabine, cytarabine and Granulocyte colony-stimulating factor (GCSF) or in combination with fludarabine, cytarabine, GCSF and a daunorubicin citrate liposome injection.
Owner:BOEHRINGER INGELHEIM INT GMBH
Slow-released injection containing nolatrexed dihydrochloride and synergist thereof
The invention relates to a slow-release injection containing nolatrexed and a synergistic agent of the nolatrexed, which consists of a slow-release microballoon sphere and a solution medium, wherein, the slow-release microballoon sphere comprises active anti-cancer ingredients and slow-release accessories; and the solution medium is a special solution medium containing a suspending agent. The active anti-cancer ingredients are antimetabolites, such as pemetrexed, rumitrexed, raltitrexed, nolatrexed, carmofur, dexrazoxane, tegafur, zalcitabine, emtricitabine, ibatabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and the like, and synergistic agents of the antimetabolites selected from topoisomerase inhibitors and / or tetrazine compounds; the slow-release accessories are selected from one of polifeprosan, di-fatty acid and decanedioic acid copolymer, polylactic acid copolymer and EVAC or the combination thereof; the viscosity of the suspending agent is 100cp to 3000cp (at the temperature of 20 DEG C to 30 DEG C) and the suspending agent is selected from carboxymethylcellulose sodium and the like. The slow-release microballoon sphere can also be prepared into a slow-release implant used for being injected or put in tumors or the surrounding of the tumors so as to enhance the effects of radiotherapy and chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Combination therapy with volasertib
Disclosed is the use of Volasertib or a salt or a hydrate thereof for treating patients suffering from acute myeloid leukemia (AML) comprising administering a high dose of Volasertib in combination with fludarabine, cytarabine and Granulocyte colony-stimulating factor (GCSF) or in combination with fludarabine, cytarabine, GCSF and a daunorubicin citrate liposome injection.
Owner:BOEHRINGER INGELHEIM INT GMBH
Tetracycline-containing medicine composition and application for same
The invention relates to a medicine composition. The medicine composition is a combination of tetracycline and at least one anti-cancer medicine selected from bortezomib, fludarabine, imatinib, cis-platinum and adriamycin amycin. The invention further discloses an application of the combinations in preparation of medicines for treating non-small cell lung cancer.
Owner:NORTHWEST A & F UNIV
Drying method for bulk drug fludarabine phosphate
InactiveCN110028538ANo degradationShorten the timeSugar derivativesChemical industryKetone solventsRoom temperature
The invention discloses a drying method for a bulk drug fludarabine phosphate. The method comprises the following steps: step 1, adding a wet fludarabine phosphate product crystallized from an aqueoussolution into a ketone solvent; step 2, conducting stirring at room temperature for 1-5 h; and step 3, conducting filtering and drying the filter cake in a vacuum oven at 50-60 DEG C for 3-5 h, so asto obtain the bulk drug, namely fludarabine phosphate. The drying method for of the bulk drug fludarabine phosphate is simple in operation, energy-saving and consumption-reducing, suitable for industrial production, and provides a new method for a bulk drug fludarabine phosphate.
Owner:连云港杰瑞药业有限公司
Combination therapy of 4-(3-(2h-1,2,3-triazo-2-yl)phenylamino)-2-((1r,2s)-2-aminocyclohexylamino)pyrimidine-5-carboxamide and fludarabine
InactiveUS20130252917A1Good effectGood treatment effectBiocideCarbohydrate active ingredientsMantle lymphomaCombination therapy
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing 4-(3-(2H-1,2,3-triazol-2-yl)phenylamino)-2-(1R,2S)-2-aminocyclohexylamino)pyrimidine-5-carboxamide, or a pharmaceutically acceptable salt thereof, and fludarabine for the treatment of cell proliferative disorders, such as undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), including diffuse large B cell lymphoma (DLBCL); mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic Lymphoma (SLL) and multiple myeloma.
Owner:ALEXION PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com