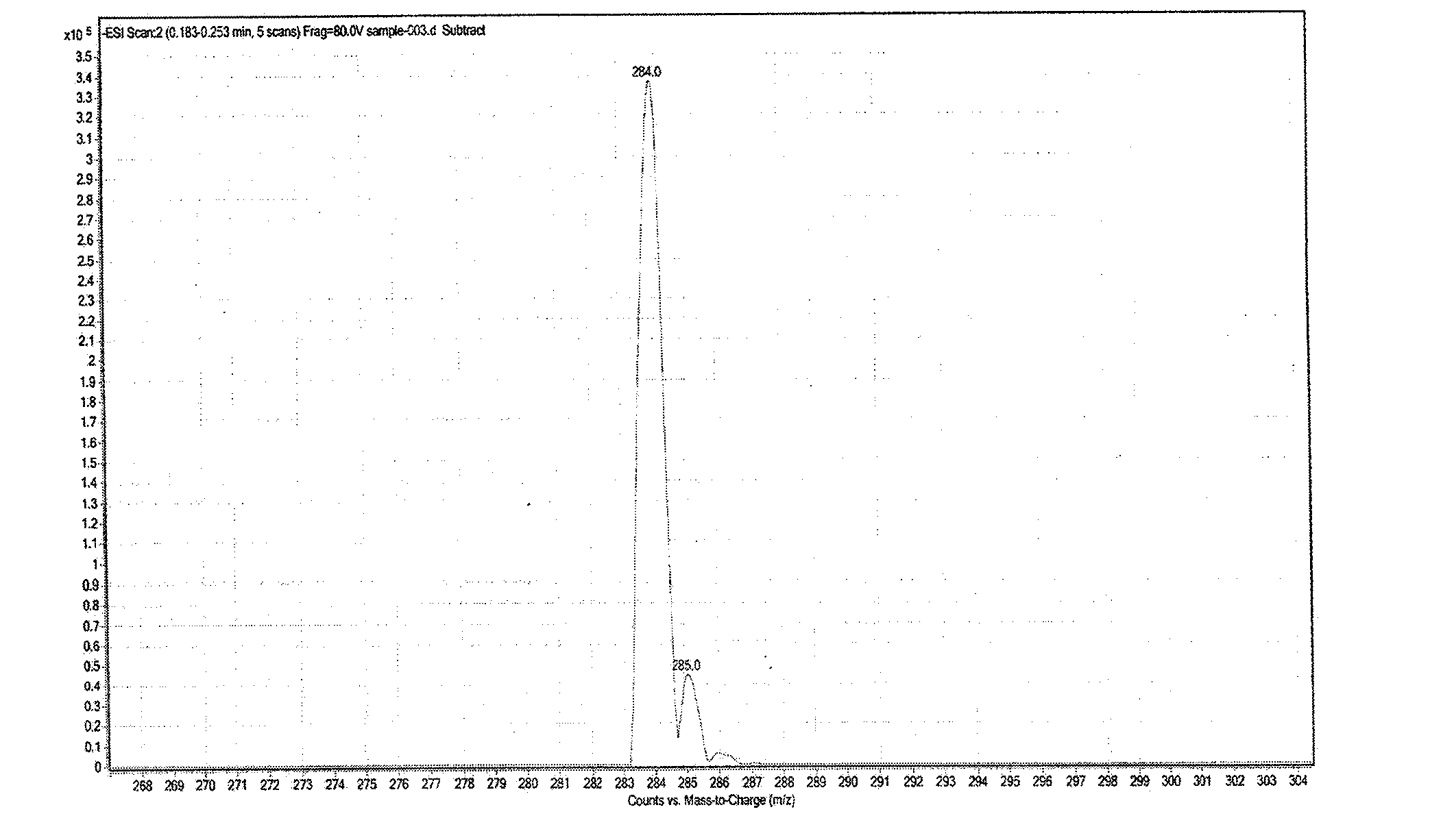
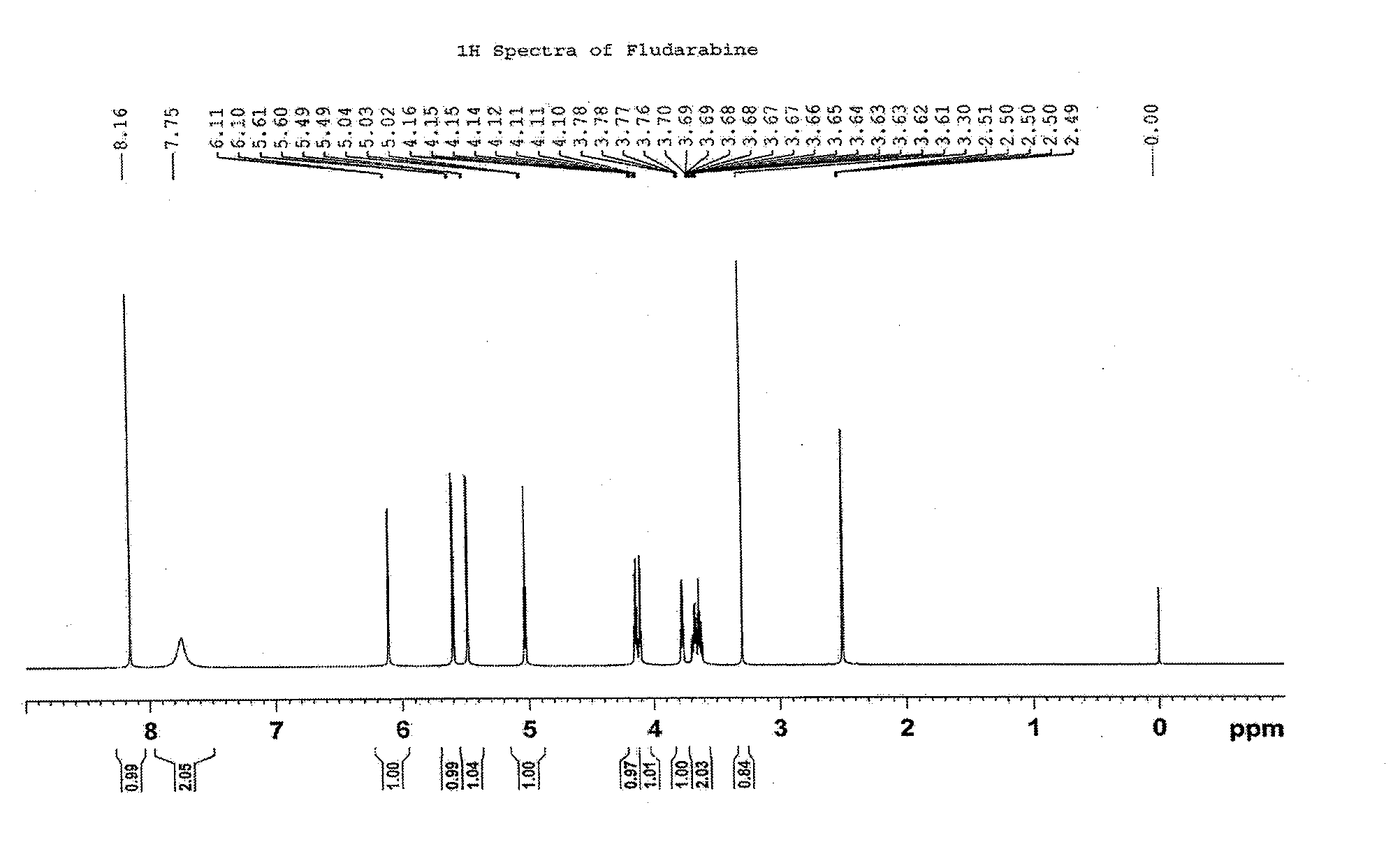
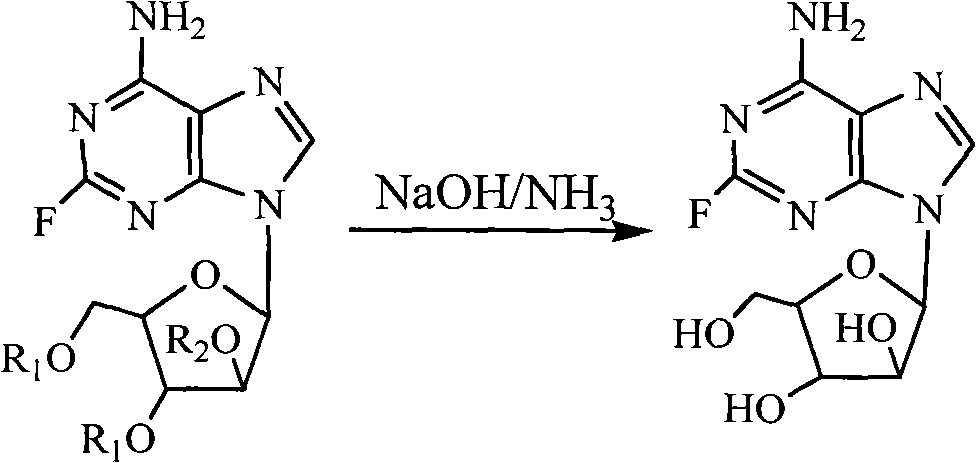
Synthesis method of fludarabine
A technology of fludarabine and its synthetic method, which is applied in the field of drug preparation, can solve the problems of being unsuitable for industrial production and harsh reaction conditions, and achieve the effects of cheap raw materials, short reaction time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 fludarabine
[0027] Add 400mL of 2.5N NaOH solution and 300mL of concentrated ammonia water into the reaction flask, put the reaction flask into an ice-water bath and cool to 0~5°C; add 4L of 2-methyltetrahydrofuran and 400mL of water into the above reaction flask, stir and cool to 0~5℃; under stirring, add 225g of 2-fluoro-9-β-D-(2′,3′,5′-tri-O-acetylarabinofuranosyl)adenine in portions; after the addition, the mixture Stir and react at 0-5°C for 2 h; after TLC (developing agent: chloroform:methanol=9:1) shows that the reaction is complete, add 35 mL of glacial acetic acid to the reaction mixture, and stir for 5-10 min. The reaction mixture was concentrated to dryness under reduced pressure, 1.5 L of water was added to the residue, stirred at room temperature for 5-10 min, and filtered to obtain crude fludarabine. The crude product was recrystallized with water and decolorized with activated carbon, and the precipitated solid was filtere...
Embodiment 2
[0028] The synthesis of embodiment 2 fludarabine
[0029] Add 400mL of 2.5N NaOH solution and 280mL of concentrated ammonia water into the reaction flask, put the reaction flask into an ice-water bath and cool to 0~5°C; add 5L of 2-methyltetrahydrofuran and 400mL of water into the above reaction flask, stir and cool to 0~5℃; 283g 2-fluoro-9-β-D-(3′,5′-di-O-acetyl-2′-O-(4-nitrobenzoyl)arabinofuranose Base) adenine was added in portions; after the addition, the mixture was stirred and reacted at 0-5°C for 2-2.5h; after TLC (developing agent: chloroform:methanol=9:1) showed that the reaction was complete, add 30mL glacial acetic acid, stirred for 5-10min. The reaction mixture was concentrated to dryness under reduced pressure, 1.2 L of water was added to the residue, stirred at room temperature for 5-10 min, and filtered to obtain crude fludarabine. The crude product was recrystallized with water, and decolorized with activated carbon, and the precipitated solid was filtered, w...
Embodiment 3
[0030] The synthesis of embodiment 3 fludarabine
[0031] Add 400mL 2.5N NaOH solution and 250mL concentrated ammonia water into the reaction flask, put the reaction flask into an ice-water bath and cool to 0~5°C; add 4000mL 2-methyltetrahydrofuran and 450mL water into the above reaction flask, stir and cool to 0~5℃; under stirring, add 225g of 2-fluoro-9-β-D-(2′,3′,5′-tri-O-acetylarabinofuranosyl)adenine in portions; after the addition, the mixture Stir and react at 0-5°C for 1.5 h; after TLC (developing agent: chloroform:methanol=9:1) shows that the reaction is complete, add 30 mL of glacial acetic acid to the reaction mixture, and stir for 5-10 min. The reaction mixture was concentrated to dryness under reduced pressure, 1.2 L of water was added to the residue, stirred at room temperature for 5-10 min, and filtered to obtain crude fludarabine. The crude product was recrystallized with water and decolorized with activated carbon, and the precipitated solid was filtered, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com