Drying method for bulk drug fludarabine phosphate
A technology of fludarabine phosphate and fludarabine phosphate wet product, which is applied in the drying field of fludarabine phosphate bulk drug, can solve problems such as the reliability of fludarabine phosphate, and achieve high safety and fast time The effect of shortening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for drying fludarabine phosphate crude drug, comprising the steps of:
[0029] Step 1, adding the bulk drug of fludarabine phosphate with larger water content to acetone with 20 times the volume;
[0030] Step 2, stirring at 15°C for 5h;
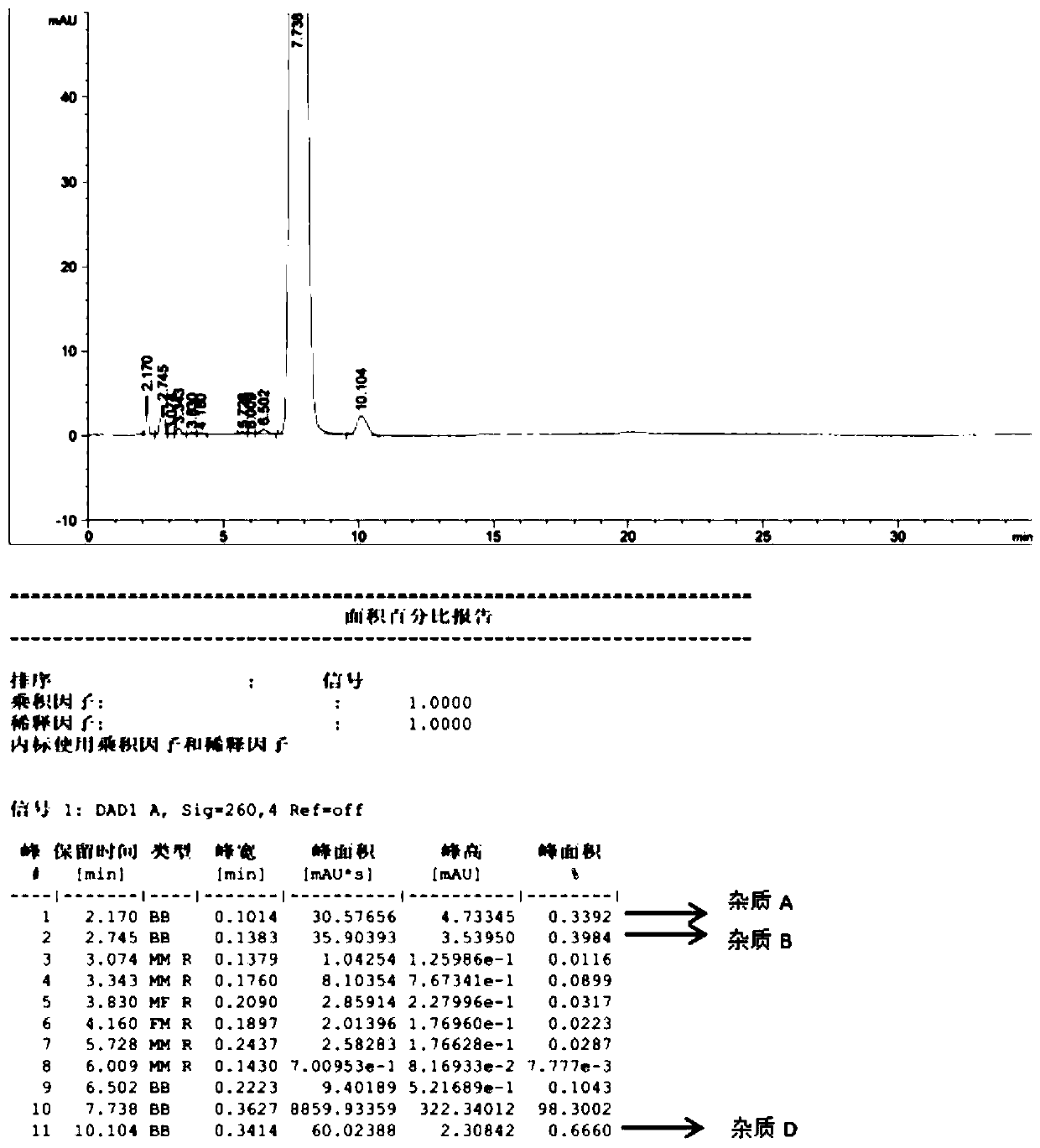
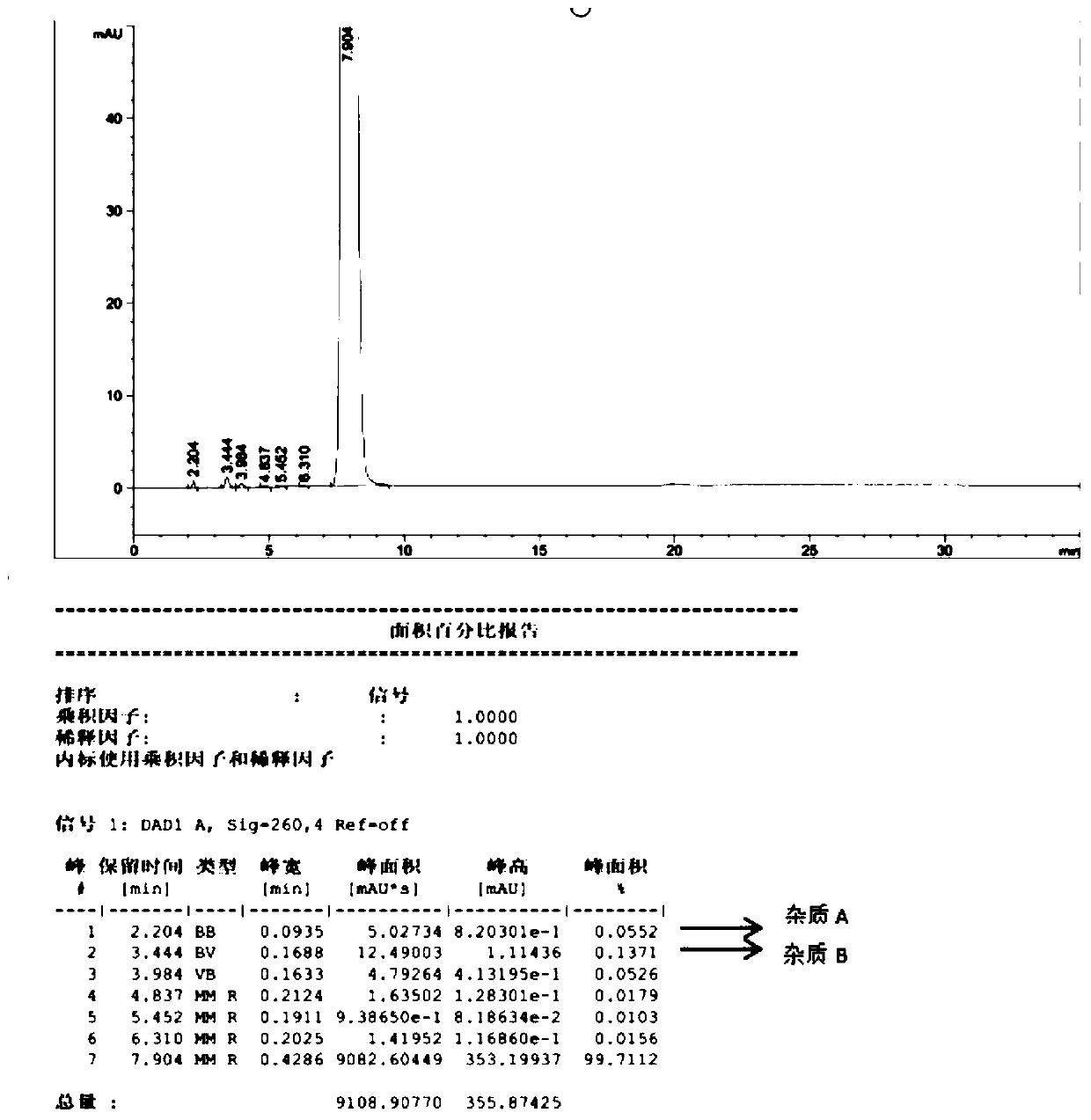
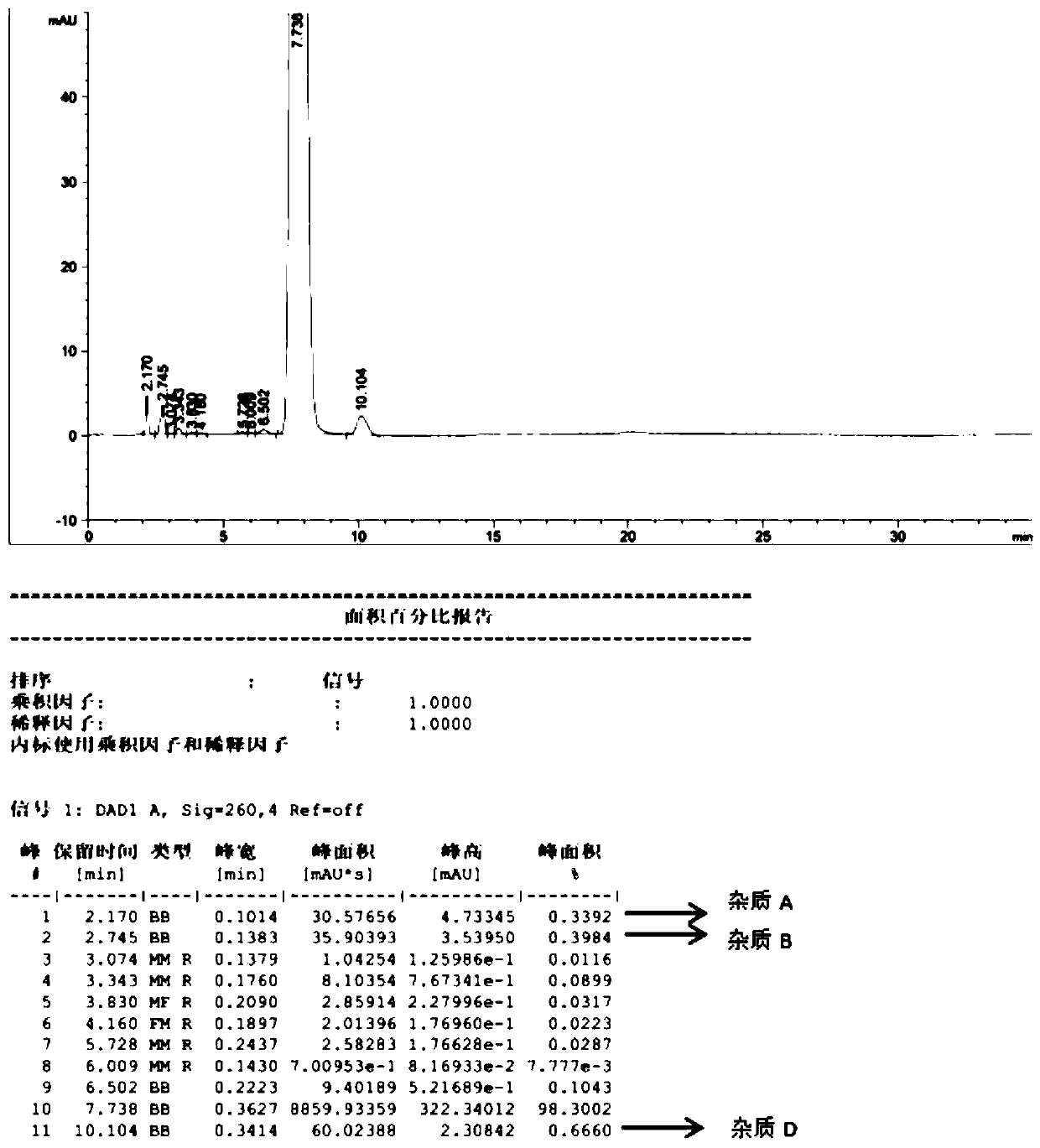
[0031] Step 3, filtering, drying the filter cake at 55° C. for 3 hours in a vacuum drying oven to obtain the fludarabine phosphate bulk drug with a water content of less than 3% (the product HPLC spectrum is shown in figure 2 ).
Embodiment 2
[0033] A method for drying fludarabine phosphate crude drug, comprising the steps of:
[0034] Step 1, adding the bulk drug of fludarabine phosphate with larger water content to acetone with 30 times the volume;
[0035] Step 2, stirring at 20°C for 3h;
[0036] Step 3, filtering, drying the filter cake in a vacuum drying oven at 50° C. for 3 hours to obtain the fludarabine phosphate raw material with a water content of less than 3%.
Embodiment 3
[0038] A method for drying fludarabine phosphate crude drug, comprising the steps of:
[0039] Step 1, adding the bulk drug of fludarabine phosphate with larger water content to acetone with 25 times the volume;
[0040] Step 2, stirring at 25°C for 5h;
[0041] Step 3, filtering, drying the filter cake in a vacuum drying oven at 60° C. for 5 hours to obtain fludarabine phosphate crude drug with a water content of less than 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com