Synthetic method of 2-fluoroadenosine
A synthetic method, fluoroadenosine technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of unfavorable industrial scale production, high cost, low synthesis efficiency, etc., to achieve industrial production, Easy operation and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
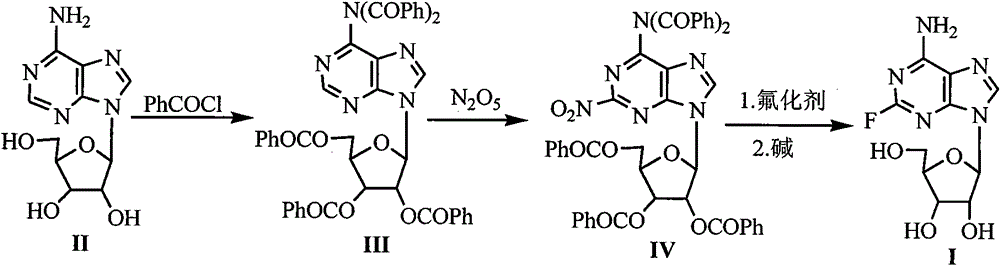
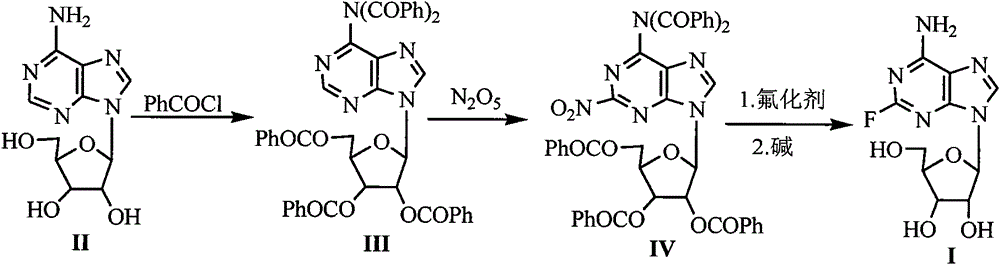
[0024] Embodiment 1 Formula (III) compound N, the preparation of N-dibenzoyl-2', 3', 5'-three-O-benzoyl adenosine
[0025] Add 40.2g (150mmol) of the compound of formula (II) adenosine, 250mL of pyridine and 147g (1046mmol) of benzoyl chloride into the reaction flask, stir and mix well. Raise the temperature of the system to 60-65°C, and stir for 4 hours to stop the reaction. After cooling, add 250 mL of ethanol, stir at room temperature for 10 minutes, evaporate the solvent under reduced pressure, add 800 mL of dichloromethane and 800 mL of water to the residue, separate Dichloromethane, the aqueous layer was extracted with dichloromethane, the dichloromethane layers were combined, washed with 200 mL of saturated brine, dried over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, and the solid was dried in vacuo to obtain 58 g, with a yield of 96%.
Embodiment 2
[0026] Example 2 Preparation of Formula (IV) Compound 2-nitro-N, N-dibenzoyl-2', 3', 5'-tri-O-benzoyl adenosine
[0027] NaZSM-5 molecular sieve powder was prepared by hydrothermal crystallization with inorganic ammonium as a template, and HZSM-5 was obtained by ammonium nitrate exchange.
[0028] Take the newly made N 2 o 5 16.2g (150mmol) was dissolved in 150mL of dichloromethane to make a concentration of 1mol / L N 2 o 5 dichloromethane solution. 94.6g (120mmol) of the compound of formula (III) was dissolved in 100mL of dichloromethane, and added to the above prepared N 2 o 5 4.5 g of HZSM-5 molecular sieves were added to the dichloromethane solution, and the reaction mixture was stirred at 45° C. for 2 h. After cooling, filter the catalyst, recover the catalyst, evaporate the solvent under reduced pressure, add 400 mL of ethyl acetate to the residue, wash the ethyl acetate layer with 400 mL of distilled water and 200 mL of saturated brine, dry over anhydrous magnesium...
Embodiment 3
[0029] The preparation of embodiment 3 formula (I) compound 2-fluoroadenosine
[0030] Add 41.7g (50mmol) of the compound of formula (IV), 5.8g (100mmol) of KF, 250mL of DMF and 0.28g (2.5mmol) of tetramethylammonium chloride into the reaction flask, and heat the reaction mixture to reflux for 5h. After cooling, filter the insoluble matter, distill off the solvent under reduced pressure, add 60mL of 2.5N NaOH solution, 350mL of 2-methyltetrahydrofuran and 45mL of water to the residue, put the reaction bottle in an ice-water bath to cool to 0-5°C, and The reaction was stirred at high temperature for 2.0 h; 6 mL of glacial acetic acid was added to the reaction mixture, and the reaction was stirred for 5-10 min. The reaction mixture was concentrated to dryness under reduced pressure, 1000 mL of ethyl acetate and 100 mL of ethanol were added to the residue, and it was placed in the refrigerator overnight, the precipitated solid was filtered and dried under reduced pressure to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com