Quality control method of compound injection liquid
A quality control method and injection technology, applied to medical preparations containing active ingredients, measuring devices, pharmaceutical formulas, etc., can solve problems such as the lack of quality control methods and the lack of technical solutions for quality control of Compound Danshen Injection, etc. To achieve safe and effective quality control, to ensure the effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Determination experiment of HPLC chromatographic elution conditions and GC chromatographic conditions
[0038] The present invention has done a lot of research experiments on HPLC chromatographic elution conditions and GC chromatographic conditions in a large number of long-term experimental studies, but cannot be repeated here. Several typical comparative experiments are selected below for description.
[0039] The HPLC chromatographic elution condition 1 is: the mobile phase composition includes that the mobile phase A is 1% acetic acid solution, and the mobile phase B is acetonitrile. For the gradient elution procedure, the specific conditions are 5% B for 0-10 min, 10% B for 120 min, 20% B50 for 35 min, 30% B for 50 min, and 40% B for 60 min; elution condition 2 is: mobile phase The composition included mobile phase A as 1% acetic acid solution and mobile phase B as acetonitrile. For the gradient elution procedure, the specific conditions are 12%B for 0-5...
Embodiment 2
[0041] The determination experiment of embodiment 2 quality control index component
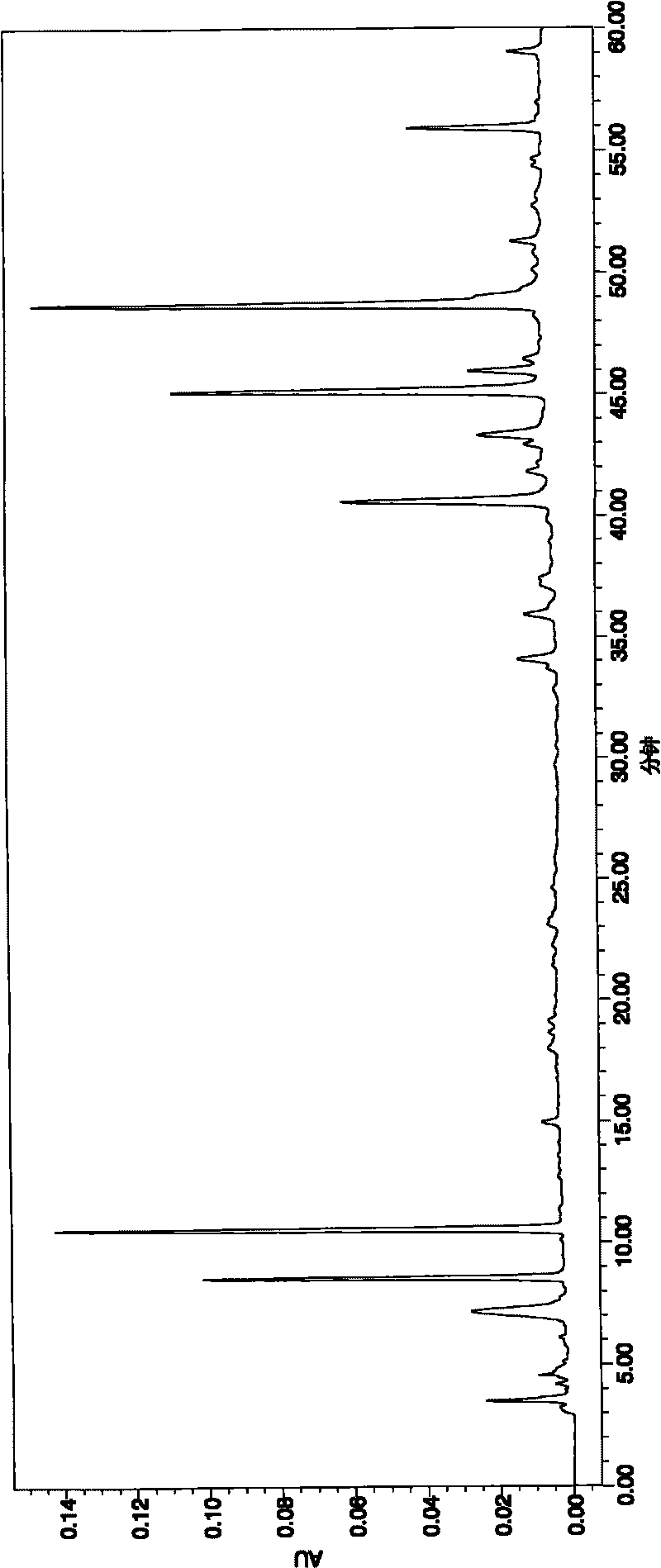
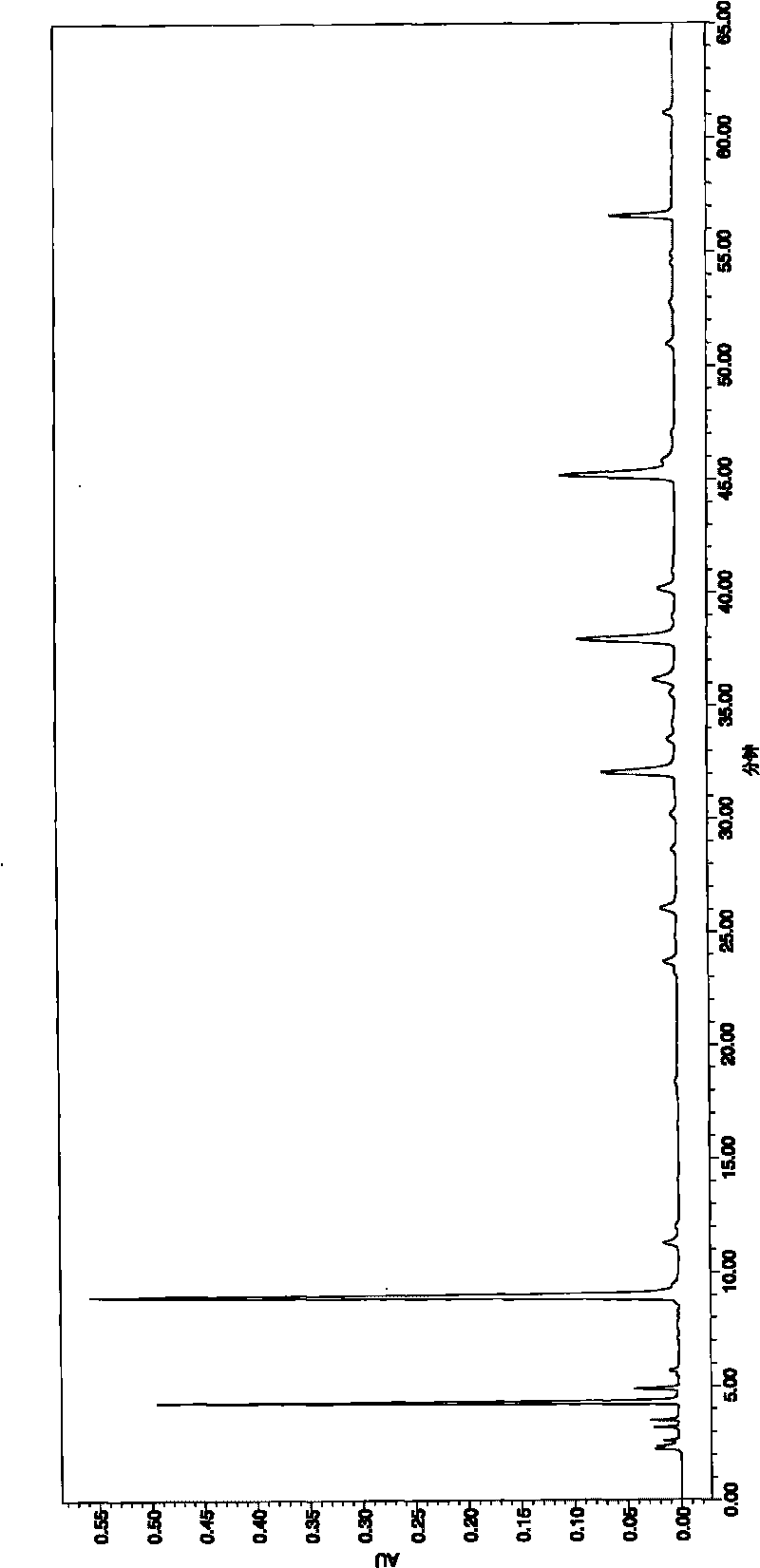
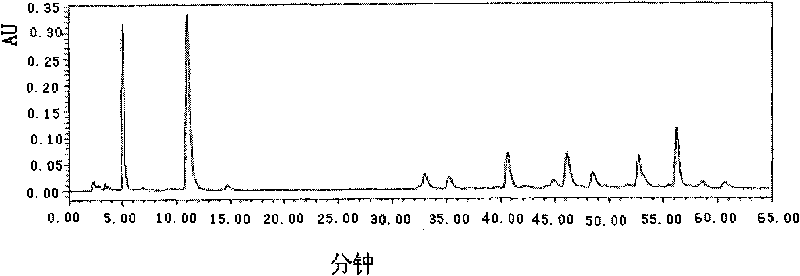
[0042] Through the anti-myocardial ischemia and anti-hypoxia experiments on randomly selected compound injections containing salvia miltiorrhiza, pharmacological studies were carried out, and the fingerprint of the compound was studied. Salvianolic acid A, the peak area sum of protocatechuic aldehyde is more than 65%, see attached Image 6 and shown in Table 1. We carry out extraction with petroleum ether, chloroform, ethyl acetate, n-butanol successively to described injection, the consumption of extraction solvent is 500ml each time, extraction 3 times, obtains 5 different polar parts, respectively petroleum ether part ( A), the chloroform part (B), the ethyl acetate part (C), the n-butanol part (D) and the water part (E) are combined, and the fingerprints of each part and the combination of parts are obtained according to the HPLC chromatographic conditions. At the same time, pharmacolog...
Embodiment 3
[0078] Embodiment 3 Determination of trans-bitterol by gas chromatography
[0079] The present invention uses temperature-programmed gas chromatography to measure trans-bitterol. The certain gas chromatographic conditions used in the present invention include the following factors: the gas chromatograph is a Shimadzu GC-2010 gas chromatograph; a gas chromatographic column: an RTX-1701 gas chromatographic column; The split ratio is 5:1, and the mobile phase is high-purity N 2 , constant flow mode, n-tetradecanol as internal standard, FID as detector, injection volume 2μL, detector temperature 250℃, flow rate 2.72mL·min -1 . The temperature program of the gas chromatographic column in the certain chromatographic conditions is: 2°C·min -1 The temperature was increased to 124 °C at a rate of 10 min; at 10 °C·min -1 The temperature was raised to 138°C at a rate of 5min; -1 The rate was raised to 220°C and held for 5min.
[0080] Qualitative and quantitative control is carried...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com