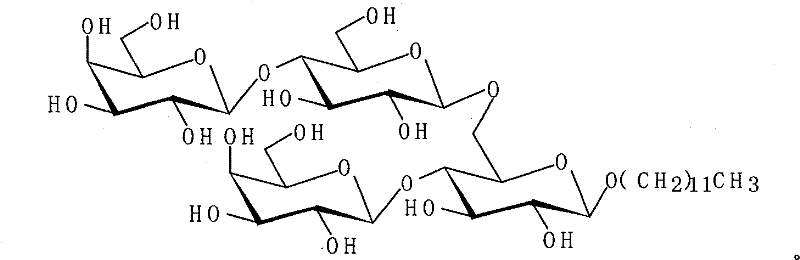
Alkyl galactoside receptor, preparation method and application thereof
A technology of heptaacetyllactoside and alkyl group is applied in the field of alkyl lactoside receptor and its preparation, which can solve the problems of complex oligosaccharide synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
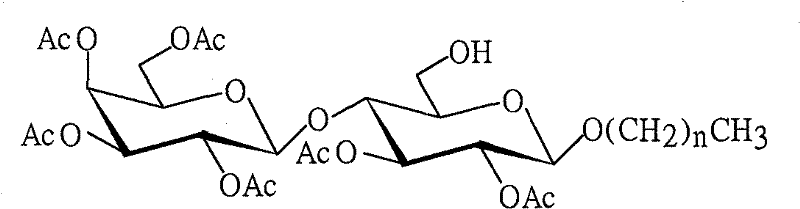
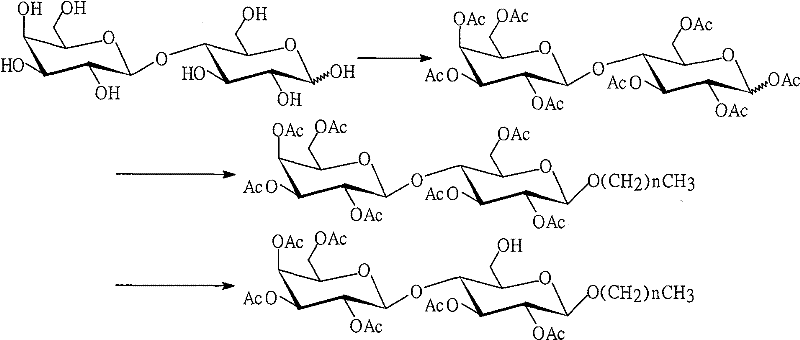
[0020] Preparation of Octyllactoside Acceptor 4
[0021] 1,2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl-(1→4)-1,2,3,6-tetra-O-acetyl-D- Synthesis of glucopyranoside 2
[0022] In a 500ml pear-shaped bottle, add 60ml of pyridine (dried with calcium hydride), and under stirring at room temperature, sequentially add lactose 1 (18.0g, 0.050mol), 60ml of acetic anhydride (excess) or 40ml of acetyl chloride (excess), and stir at room temperature for reaction 12 After the reaction was completed, 200ml of ethyl acetate was added, followed by washing with saturated brine, 1:1 hydrochloric acid solution, saturated sodium bicarbonate solution, and saturated brine to neutrality, and then washing with anhydrous sodium sulfate dry. After filtration, the solvent was recovered from the filtrate under reduced pressure, and finally a colloidal solid 2 (32.91 g) was obtained with a yield of 97%.
[0023] 2. Octyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl-β- Synthesi...
Embodiment 2
[0035]Preparation of dodecyllactoside acceptor 6
[0036] 1. Dodecyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl- Synthesis of β-D-glucopyranoside 5
[0037] In a 250ml pear-shaped bottle, add 2 (20.4g, 0.03mol) and dodecanol (5.6g, 0.03mol) in sequence, add a stirrer, vacuumize for half an hour, inject 150ml of dry dichloromethane, stir, drop Add 15ml of boron trifluoride ether solution, react at room temperature for 24 hours, add 150ml of dichloromethane, and wash with saturated sodium bicarbonate solution and brine successively until neutral. The solvent was recovered under reduced pressure, the sample was dissolved in dichloromethane and refined on a silica gel column. First, the dodecanol was washed out with petroleum ether / ethyl acetate (6 / 1), and then the dodecyl alcohol was washed out with petroleum ether / ethyl acetate (3 / 1). The eluent was used to recover raw material 2 (9.6g) to obtain product 5 (11.1g).
[0038] [α] D =-2.5° (c=1.0, CHCl...
Embodiment 3
[0049] Preparation of cetyl lactosyl acceptor 8
[0050] 1. Hexadecyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl-(1→4)-2,3,6-tri-O-acetyl- Synthesis of β-D-glucopyranoside 7
[0051] In a 250ml pear-shaped bottle, add 2 (20.4g, 0.03mol) and cetyl alcohol (7.3g, 0.03mol) in sequence, add a stirrer, vacuumize for half an hour, inject 150ml of dry toluene, stir, and drop three Boron fluoride ether solution 15ml, react at room temperature for 24 hours, add toluene 150ml, wash with saturated sodium bicarbonate solution and brine successively until neutral. Recover the solvent under reduced pressure, dissolve the sample in dichloromethane and refine it on a silica gel column, first wash out the cetyl alcohol with petroleum ether / ethyl acetate (7 / 1), and then use petroleum ether / ethyl acetate (4 / 1) as The eluent was used to recover raw material 2 (9.3g) to obtain product 7 (12.8g).
[0052] [α] D =-4.5° (c=1.0, CHCl3);
[0053] 1 H NMR (400MHz, CDCl3): δ5.31 (dd, 1H, J = 1.0, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com