A new peptide deformylase inhibitor compound and manufacturing process thereof
A compound, methyl technology, applied in the field of new antibacterial compounds, can solve the problem of not producing compounds and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
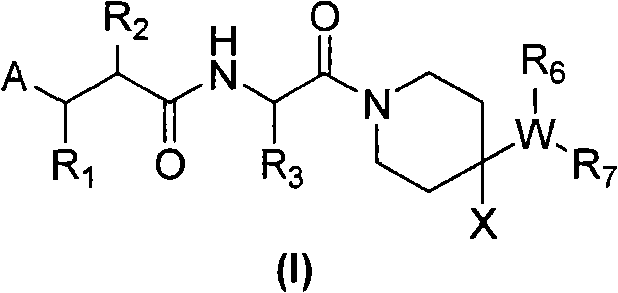
[0250] (R)-2-Cyclopentylmethyl-N 1 -{(S)-2,2-Dimethyl-1-[4-(4-methyl-benzylamino)-piperidine-1-carbonyl]-propyl}-N 4 -Hydroxy-succinic acid amide
[0251]
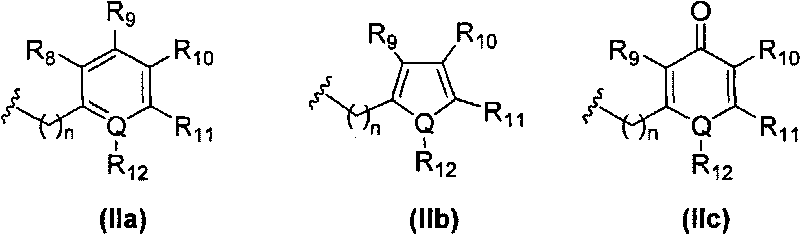
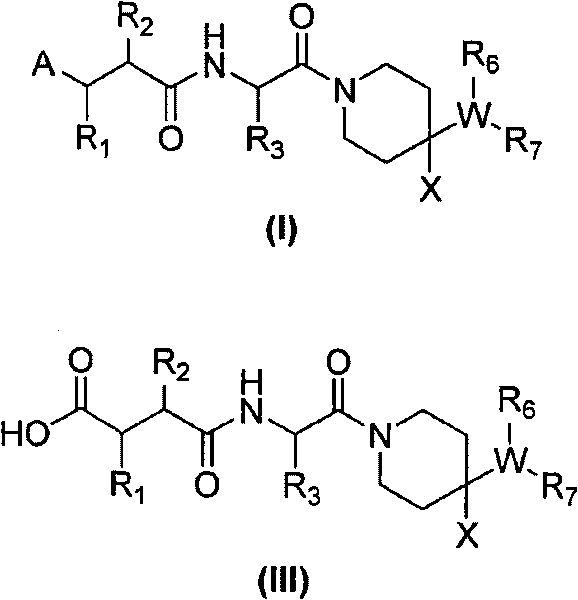
[0252] The title compound was prepared from (R)-2-cyclopentylmethyl-succinic acid 4-tert-butyl ester 5-a according to general method V (R 2 = Cyclopentylmethyl, R 13 = tert-butyl) and [1-((S)-2-amino-3,3-dimethyl-butyryl)-piperidin-4-yl]-(4-methyl-benzyl)-amino Benzyl formate hydrochloride 1-g (prepared from general method I. R 3 = tert-butyl, R 6 = benzyloxycarbonyl, R 8 =R 9 =R 11 =R 12 =X=H,R 10 =Me, Q=C, n=1).
[0253] 1 H-NMR (CDCl 3 ): δ6.93-7.20(m, 4H), 6.10-6.22(m, 1H), 4.96-5.06(m, 1H), 4.72-4.93(m, 2H), 4.61-4.71(m, 1H), 4.05 -4.35(m, 4H), 3.71-3.85(s, 2H), 2.70-2.91(m, 1H), 2.42-2.62(m, 2H), 2.33(s, 3H), 1.31-1.84(m, 12H) , 1.00 (m, 9H).
Embodiment 2
[0255] (R)-2-cyclopentylmethyl-N-{(S)-2,2-dimethyl-1-[4-(4-methyl-benzylamino)-piperidine-1-carbonyl ]-Propyl}-3-(formyl-hydroxy-amino)-propionamide
[0256]
[0257] The title compound was prepared from (R)-3-(benzyloxy-formyl-amino)-2-cyclopentylmethyl-propionic acid 6-a (R) according to general method VI 2 = Cyclopentylmethyl, R 13 = benzyl) and [1-((S)-2-amino-3,3-dimethyl-butyryl)-piperidin-4-yl]-(4-methyl-benzyl)-amino Benzyl formate hydrochloride 1-g (prepared by general method I. R 3 = tert-butyl, R 6 = benzyloxycarbonyl, R 8 = R 9 = R 11 = R 12 =X=H,R 10 =Me, Q=C, n=1).
[0258] 1 H-NMR (CDCl 3 ): δ8.39(s, 0.4H), 7.80(s, 0.6H), 7.18-7.22(m, 2H), 7.11-7.17(m, 2H), 4.87-4.97(m, 1H), 4.20-4.54 (m, 1H), 3.79(s, 2H), 3.73-4.14(m, 1H), 3.42-3.58(m, 1H), 2.93-3.17(m, 1H), 2.63-2.90(m, 3H), 2.34 (s, 3H), 1.19-2.06 (m, 14H), 0.87-1.13 (m, 11H).
Embodiment 3
[0260] (R)-N-{(S)-1-[4-(4-cyano-benzylamino)-piperidine-1-carbonyl]-2,2-dimethyl-propyl}-2- Cyclopentylmethyl-3-(formyl-hydroxy-amino)-propionamide
[0261]
[0262] The title compound was prepared from (R)-3-(benzyloxy-formyl-amino)-2-cyclopentylmethyl-propionic acid 6-a (R) according to general method VI 2 = Cyclopentylmethyl, R 13 = benzyl) and [1-((S)-2-amino-3,3-dimethyl-butyryl)-piperidin-4-yl]-(4-cyano-benzyl)-amino Benzyl formate hydrochloride 1-g (prepared by general method I. R 3 = tert-butyl, R 6 = benzyloxycarbonyl, R 8 = R 9 = R 11 = R 12 =X=H,R 10 =CN, Q=C, n=1).
[0263] 1 H-NMR (CDCl 3 ): δ8.39(s, 0.3H), 7.81(s, 0.7H), 7.57-7.65(m, 2H), 7.40-7.49(m, 2H), 4.85-4.97(m, 1H), 4.18-4.56 (m, 1H), 3.96-4.16(m, 1H), 3.89(s, 2H), 3.40-3.57(m, 1H), 2.95-3.18(m, 1H), 2.65-2.92(m, 3H), 1.17 -2.06 (m, 14H), 0.83-1.13 (m, 11H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com