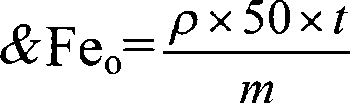Mensuration method for amorphous Fe in soil
A determination method and amorphous technology, which can be used in material analysis by observing the influence of chemical indicators and by chemical reaction of materials, etc., can solve problems such as poor selectivity, and achieve the effect of overcoming the high experimental results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
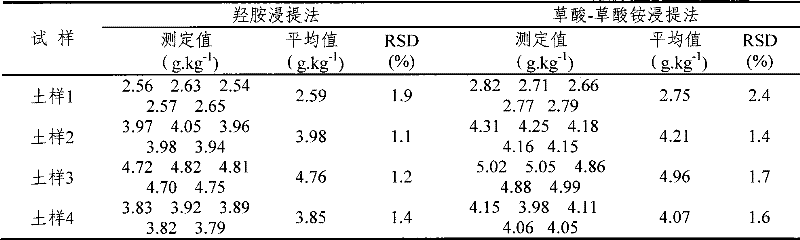
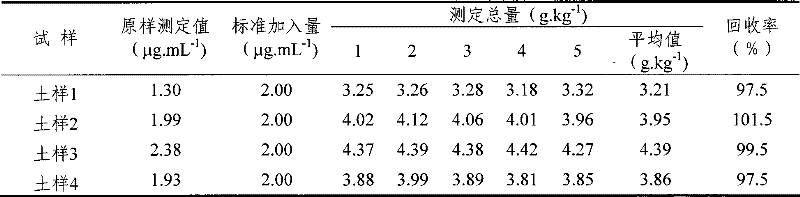
[0037] Determination method: add hydrochloric acid solution and hydroxylamine hydrochloride solution to the soil sample, stir for 60 seconds, then place at room temperature for 2 hours and filter; pipette 20ml of the filtered extract and add 2.00mL of hydroxylamine hydrochloride solution, shake well, and place for 10 minutes to make Fe(III ) are all reduced to Fe(II), then add sodium acetate solution to adjust the pH of the extract to 4-5, add the color developer o-phenanthroline after the pH value is adjusted, mix and set the volume at room temperature for 60 minutes to make it fully Color development; finally, the content of amorphous iron and iron in the soil sample was measured on a 520nm wavelength spectrophotometer; hydrochloric acid solution and hydroxylamine hydrochloride solution were added to the soil sample, and the ratio was 1:40:40 in parts by weight. The soil sample is collected fresh soil, and plant residues can be seen in the soil sample, which is air-dried and ...
Embodiment 3
[0039] Determination method: add hydrochloric acid solution and hydroxylamine hydrochloride solution to the soil sample, stir for 40 seconds, place at room temperature for 1.5 hours and filter; pipette 15ml of filtered extract and add 1.50mL hydroxylamine hydrochloride solution, shake well, place for 8 minutes to make Fe(III ) are all reduced to Fe(II), then add sodium acetate solution to adjust the pH of the extract to 5-6, add the color developer o-phenanthroline after the pH value is adjusted, mix and set the volume at room temperature for 40 minutes to make it fully Color development; finally measure the content of amorphous iron and iron in the soil sample on a 520nm wavelength spectrophotometer; add hydrochloric acid solution and hydroxylamine hydrochloride solution to the soil sample, which is 1:50:50 in parts by weight. The soil sample is collected fresh soil, and plant residues can be seen in the soil sample, which is air-dried and passed through a 0.1mm sieve.
Embodiment 4
[0041] Determination method: add hydrochloric acid solution and hydroxylamine hydrochloride solution to the soil sample, stir for 50 seconds, place at room temperature for 2 hours and filter; pipette 5ml of the filtered extract and add 1.80mL of hydroxylamine hydrochloride solution, shake well, place for 6 minutes to make Fe(III ) are all reduced to Fe(II), then add sodium acetate solution to adjust the pH of the extract to 4-5, add the color developer o-phenanthroline after the pH value is adjusted, mix and set the volume at room temperature for 50 minutes to make it fully Color development; finally measure the content of amorphous iron and iron in the soil sample on a 520nm wavelength spectrophotometer; add hydrochloric acid solution and hydroxylamine hydrochloride solution to the soil sample, which is 1:30:30 in parts by weight. The soil sample is collected fresh soil, and plant residues can be seen in the soil sample, which is air-dried and passed through a 0.15mm sieve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com