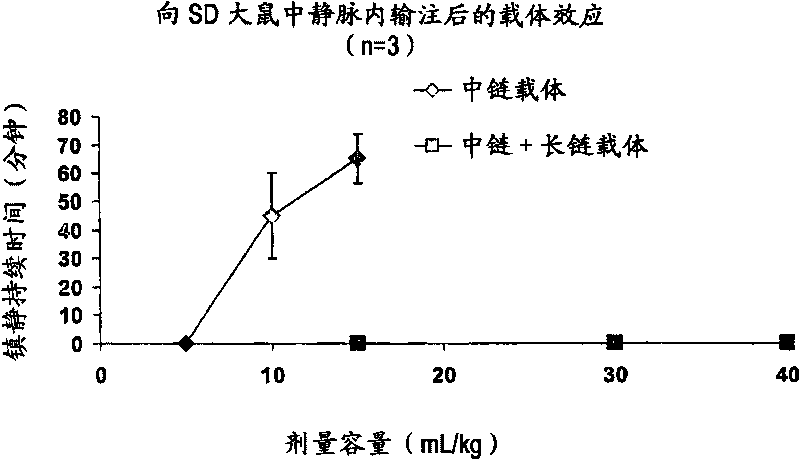
Drug formulations having long and medium chain triglycerides
A technology of long-chain triglycerides and medium-chain triglycerides, which is applied in the field of emulsified preparations of ansamycin, and can solve the problem of no combination of medium-chain triglycerides and ansamycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Preparation of 17-AAG; Alternative 1
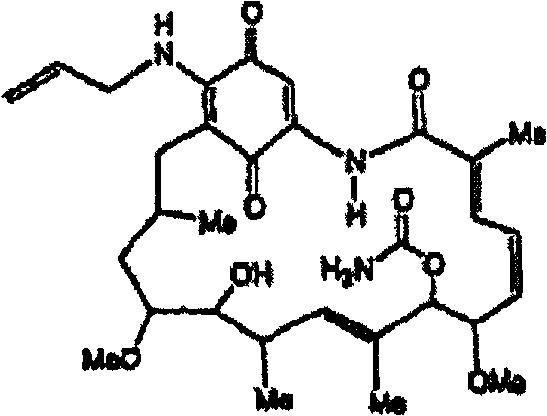
[0084] To 45.0 g (80.4 mmol) of geldanamycin dissolved in 1.45 L of dry THF in a 2 L dry flask was added dropwise 36.0 mL (470 mmol) of allylamine dissolved in 50 mL of dry THF over 30 minutes . The reaction mixture was stirred at room temperature under nitrogen for 4 hours until TLC analysis indicated that the reaction was complete [GDM: bright yellow: Rf = 0.40; (5% MeOH-95% CHCl 3 ); 17-AAG: purple: Rf = 0.42 (5% MeOH-95% CHCl 3 )]. The solvent was removed by rotary evaporation, and the crude product was heated at 25 °C in 420 mL H 2 A slurry was formed in O:EtOH (90:10), then filtered, and dried at 45 °C for 8 hours to give 40.9 g (66.4 mmol) of 17-AAG as purple crystals (82.6% yield, purity monitored by HPLC at 254 nm >98%). The melting point measured by differential scanning colorimetry (DSC) is 206-212°C. 1 The results obtained by H NMR and HPLC were consistent with the desired product.
Embodiment 2
[0085] Embodiment 2: Preparation of low melting point type 17-AAG
[0086] An alternative method of purification is to dissolve the crude 17-AAG from Example 1 in 800 mL of 2-propanol (isopropanol) at 80 °C and then cool to room temperature. Filtration followed by drying at 45° C. for 8 hours afforded 44.6 g (72.36 mmol) of 17-AAG as purple crystals (90% yield, >99% purity by HPLC at 254 nm). The melting point measured by differential scanning colorimetry (DSC) is 147-175°C. 1 The results obtained by H NMR and HPLC were consistent with the desired product.
Embodiment 3
[0087] Example 3: Preparation of low melting point type 17-AAG, alternative 1
[0088] An alternative purification method is to dissolve the product 17-AAG of Example 2 in 400 mL of H 2O:EtOH (90:10) was slurried at 25°C, filtered, and dried at 45°C for 8 hours to afford 42.4 g (68.6 mmol) of purple crystals of 17-AAG (95% yield by HPLC at 254 nm Monitored for purity >99%). The melting point is 147-175°C. 1 The results obtained by H NMR and HPLC were consistent with the desired product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com