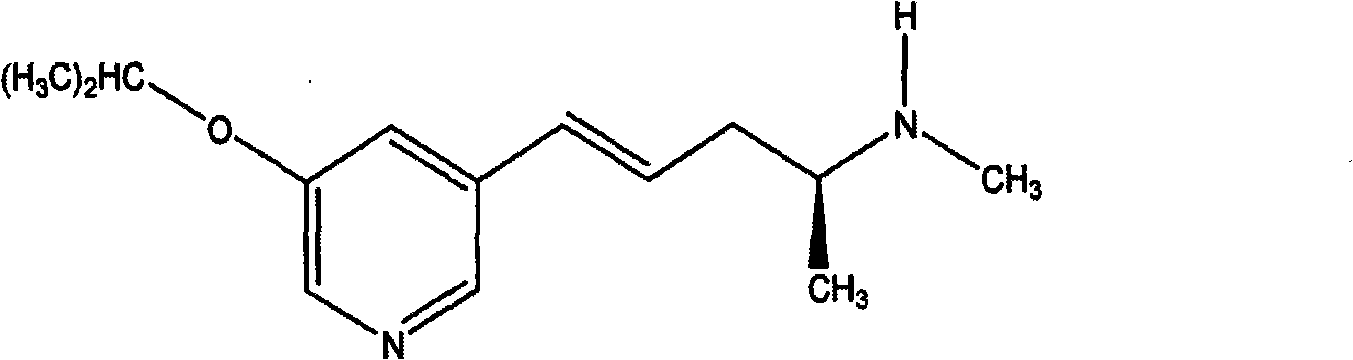
Transdermal administration of (2S)-(4E)-n-methyl-5-(3-(5-isopropoxypyridin)yl)-4-penten-2-amine
A kind of technology of propoxypyridine and methyl, applied in (2S)-(4E)-N-methyl-5-(3-(5-isopropoxypyridyl)yl)-4-pentene-2 -In the field of transdermal administration of amines, it is possible to solve problems such as administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] II. Preparation of active ingredients
[0045] (2S)-(4E)-N-Methyl-5-(3-(5-isopropoxypyridinyl)-4-penten-2-amine can be synthesized using, for example, the method described in U.S. Patent No. 6,958,399 method, the aforementioned patents are incorporated herein by reference. (2S)-(4E)-N-methyl-5-(3-(5-isopropoxypyridinyl) base)-4-pentene-2-amine salt can be synthesized by (2S)-(4E )-N-methyl-5-(3-(5-isopropoxypyridinyl)-4-penten-2-amine is combined with various inorganic acids and organic acids in a suitable solvent, as As set forth in US Patent No. 6,432,954 and PCT WO 06 / 053082, each of which is incorporated herein by reference. (2S)-(4E)-N-methyl-5-(3-(5-isopropoxypyridinyl) base)-4-penten-2-amine galactaric acid half salt (hemigalactarate) can Prepared using techniques described in US Patent No. 6,958,399, which is incorporated herein by reference.
[0046] III. Transdermal Compositions
[0047] Compositions for transdermal administration include (2S)-(4E)-N-meth...
Embodiment 1
[0152] Determination of Drug Absorption
[0153] The ability of transdermal compositions to deliver the active ingredient at pharmaceutically effective levels to a subject in need of such treatment can be evaluated by performing an in vitro drug permeation test on the abdominal skin of a guinea pig. These experiments can be performed using a diffusion chamber, such as a Franz Vertical Diffusion Cell. Skin can be obtained from 8-16 month old, ideally shaved and undamaged female guinea pigs. Sections of full-thickness abdominal skin can be surgically incised and placed with the epidermis facing up between sections of the vertical diffusion pool. The transdermal device is applied on the epithelial layer, and at the same time, the dermal layer is contacted with a receptor solution in PBS (or other suitable isotonic solution), with or without the addition of an appropriate amount of polyoxyethylene 20 oleyl ether (Oleth 20 ) or other surfactants to prevent foaming (pH 7.4). Th...
Embodiment 2
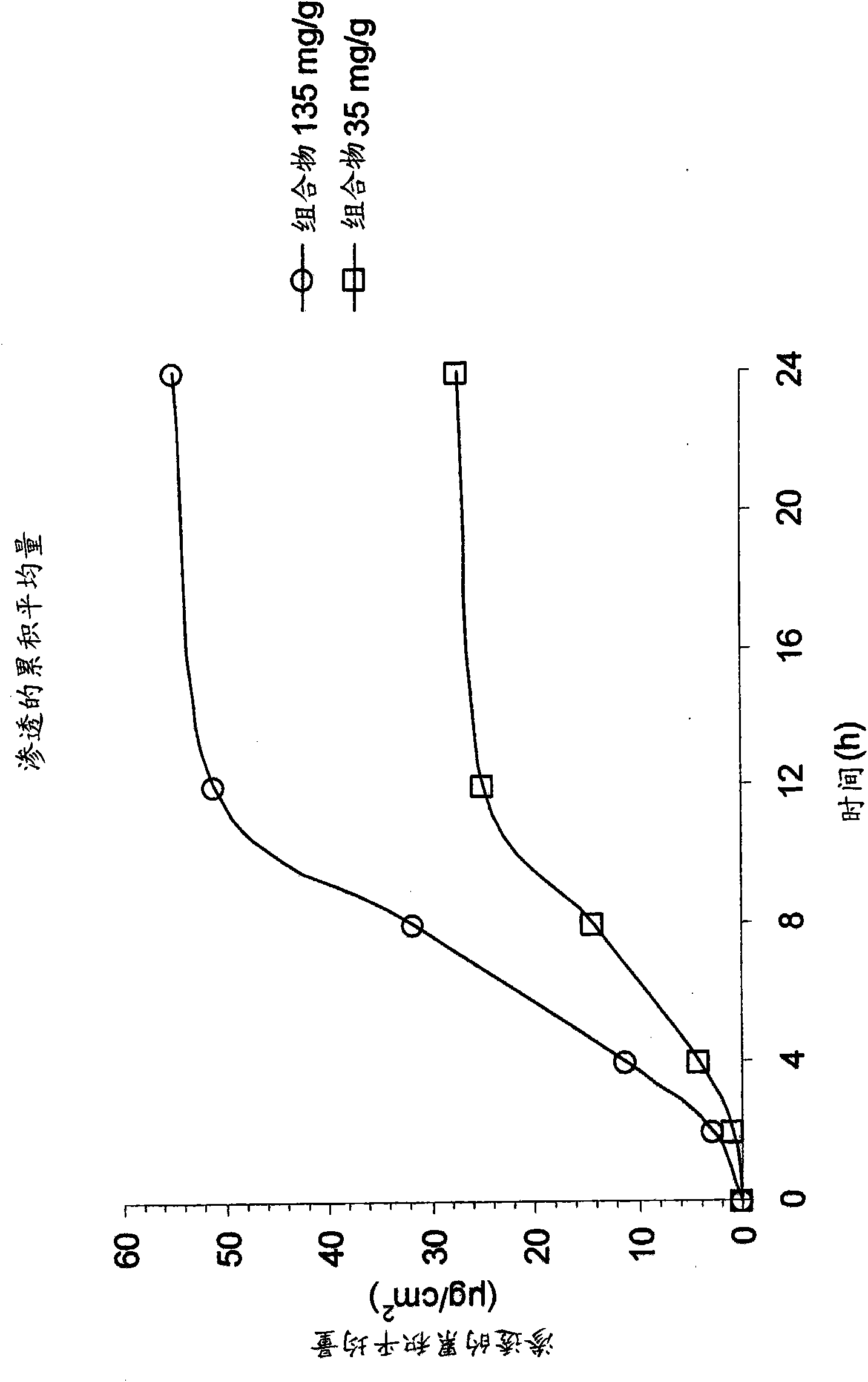
[0158] In Vitro Skin Penetration Studies Using Pig Ears
[0159] Bronaugh diffusion cells are used in in vitro permeation studies, which are performed to determine 14 C-The flux of active ingredient penetration (ie, the speed at which the compound penetrates through the skin) and the total cumulative amount. Compounds were dissolved in water at concentrations of 35 and 135 mg / g. The full-thickness skin of a pig's ear (the thickness of the skin is taken to be about 900 μm) was used as a membrane. The receiving chamber contained phosphate buffered saline (pH 7.4) and was maintained at 32°C. Apply the compound solution to the stratum corneum. Receptor chamber samples (2, 4, 8, 12 and 14h) were analyzed by liquid scintillation counting. Transdermal flux (μg / cm 2 / h) can be determined from the steady state slope of a plot of the cumulative amount of active ingredient permeated through the skin versus time. After the steady state has been established, the linear portion of t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap