Benzothiophene derivative, preparation method and application thereof
A thiophene and benzo technology, applied in the field of hepatitis C virus replication inhibitors, can solve problems such as adverse reactions, hematological toxicity, and successful cure rate of less than 10%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
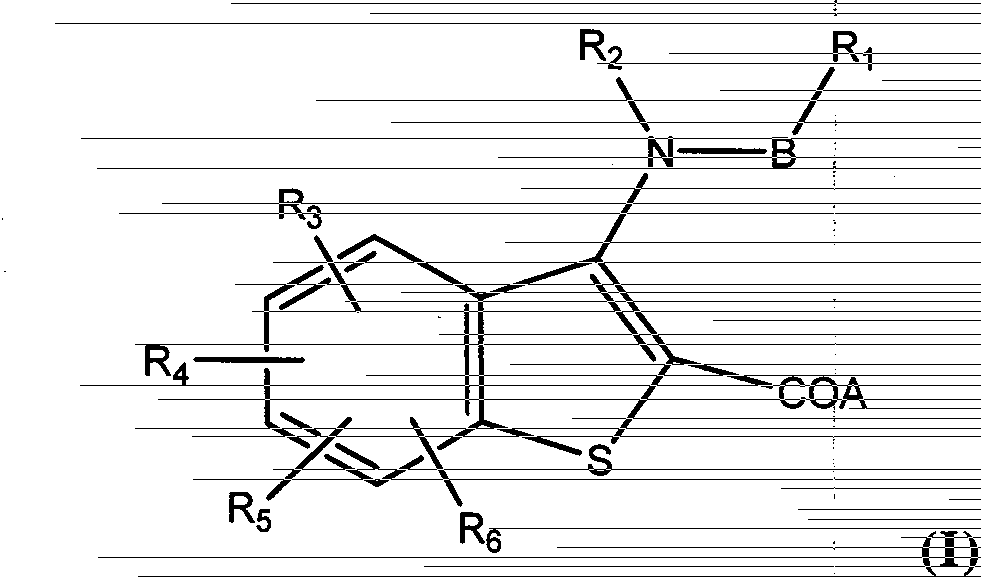
[0086] Example 1 Compound Ia: 3-(nitrogen-isopropyl-4-methylcyclohexylcarboxamido)-7-phenylbenzo[b]thiophene-2-carboxylic acid
[0087]
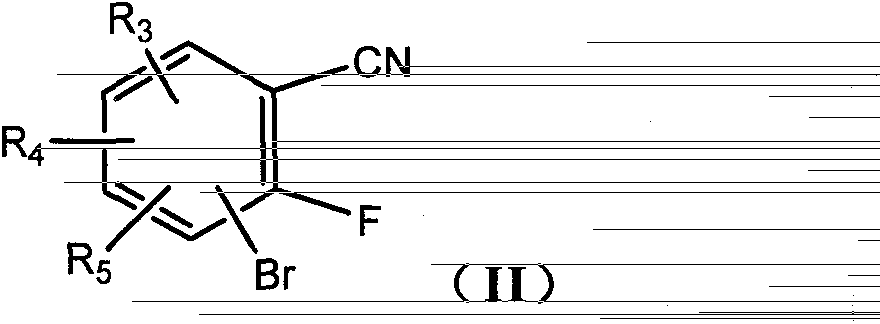
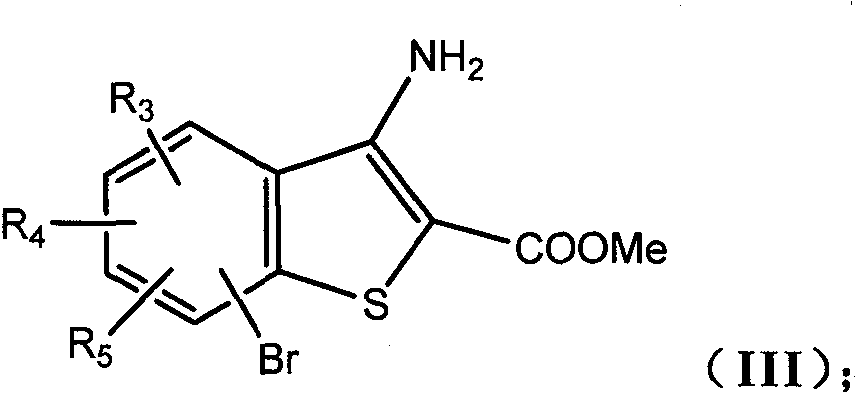
[0088] First, intermediate IIIa: methyl 3-amino-7-bromobenzo[b]thiophene-2-carboxylate should be synthesized, and the target compound should be synthesized from intermediate IIIa.
[0089]
[0090] According to the synthetic route (1), under the protection of nitrogen, 3-bromo-2-fluorobenzocyanide (5.0g, 25.1mmol) was dissolved in 20.0ml of DMF, and sodium carbonate (5.3g, 50.0mmol) was added to cool to 0°C. Methyl thioglycolate (2.9 g, 27.5 mmol) was added dropwise with stirring, and the reaction solution was raised to room temperature and stirred overnight. After adding water, a white solid was precipitated, filtered, washed with water and petroleum ether, and dried to obtain a white powder (6.4 g), with a yield of 89.5%.
[0091]1 H NMR (CDCl 3 , 300MHz) δ7.64-7.60(m, 2H), 7.29-7.24(m, 1H), 5.86(br, 2H), 3.90(s, 3H); ESI-MS m / z 28...
Embodiment 2
[0106] Example 2 Compound Ib: 3-(nitrogen-isopropyl-4-methylcyclohexylcarboxamido)-6-phenylbenzo[b]thiophene-2-carboxylic acid
[0107]
[0108] Synthesis of Intermediate IIIb
[0109] 3-Amino-6-bromobenzo[b]thiophene-2-carboxylic acid methyl ester
[0110]
[0111] According to the synthetic route (1), under nitrogen protection, 4-bromo-2-fluorobenzocyanide (5.0g, 25.1mmol) was dissolved in 20.0ml DMF, and sodium carbonate (5.3g, 50.0mmol) was added to cool to 0°C, and stirred Methyl thioglycolate (2.9 g, 27.5 mmol) was added dropwise under the conditions of , the reaction solution was raised to room temperature, and stirred overnight. After adding water, a white solid was precipitated, filtered, washed with water and petroleum ether, and dried to obtain a white powder (6.0 g), with a yield of 83.9%.
[0112] 1 H NMR (CDCl 3 , 300MHz) δ7.88(s, 1H), 7.48(s, 2H), 5.88(s, 2H), 3.89(s, 3H); ESI-MS m / z 284(M + -H + ).
[0113] The following steps follow the method for...
Embodiment 3
[0115] Example 3 Compound Ic: 3-(nitrogen-ethyl-4-methylcyclohexylcarboxamido)-6-phenylbenzo[b]thiophene-2-carboxylic acid
[0116]
[0117] Compound Ic can be prepared according to the method for preparing compound Ib in Example 2.
[0118] 1 H NMR (DMSO-d6, 300MHz) δ8.40(s, 1H), 7.86-7.74(m, 4H), 7.53-7.41(m, 3H), 3.82-3.58(m, 2H), 2.10-2.02(m , 1H), 1.84-1.80(m, 3H), 1.66-1.63(m, 3H), 1.43-1.24(m, 6H), 0.83(d, J=6.6Hz, 3H); ESI-MS m / z 422 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com