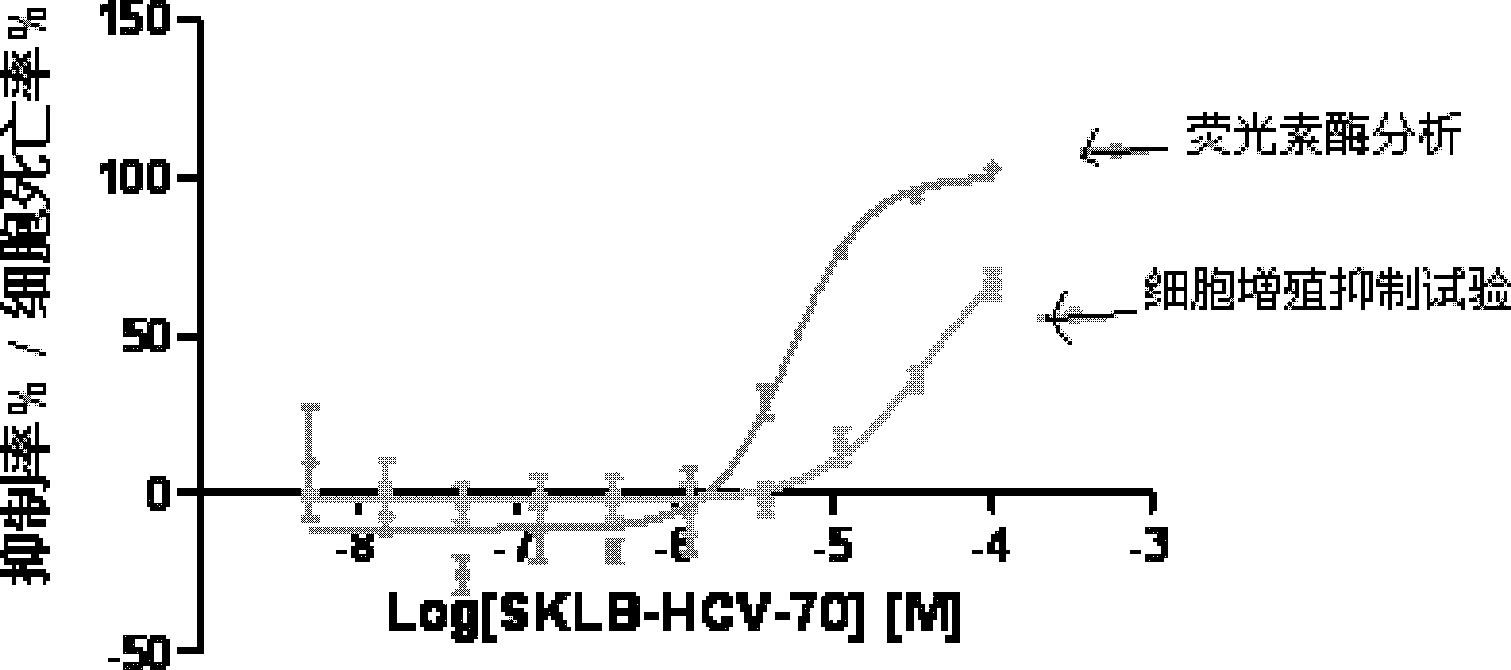
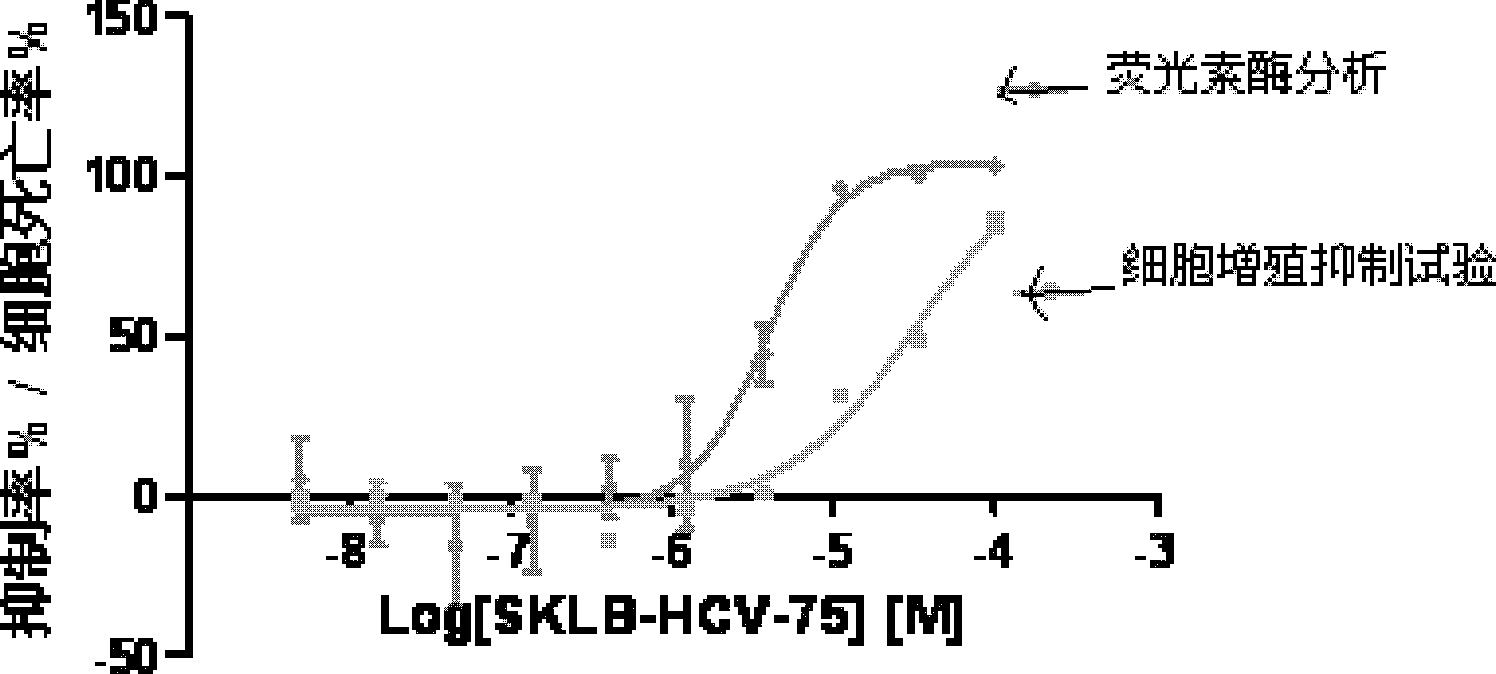
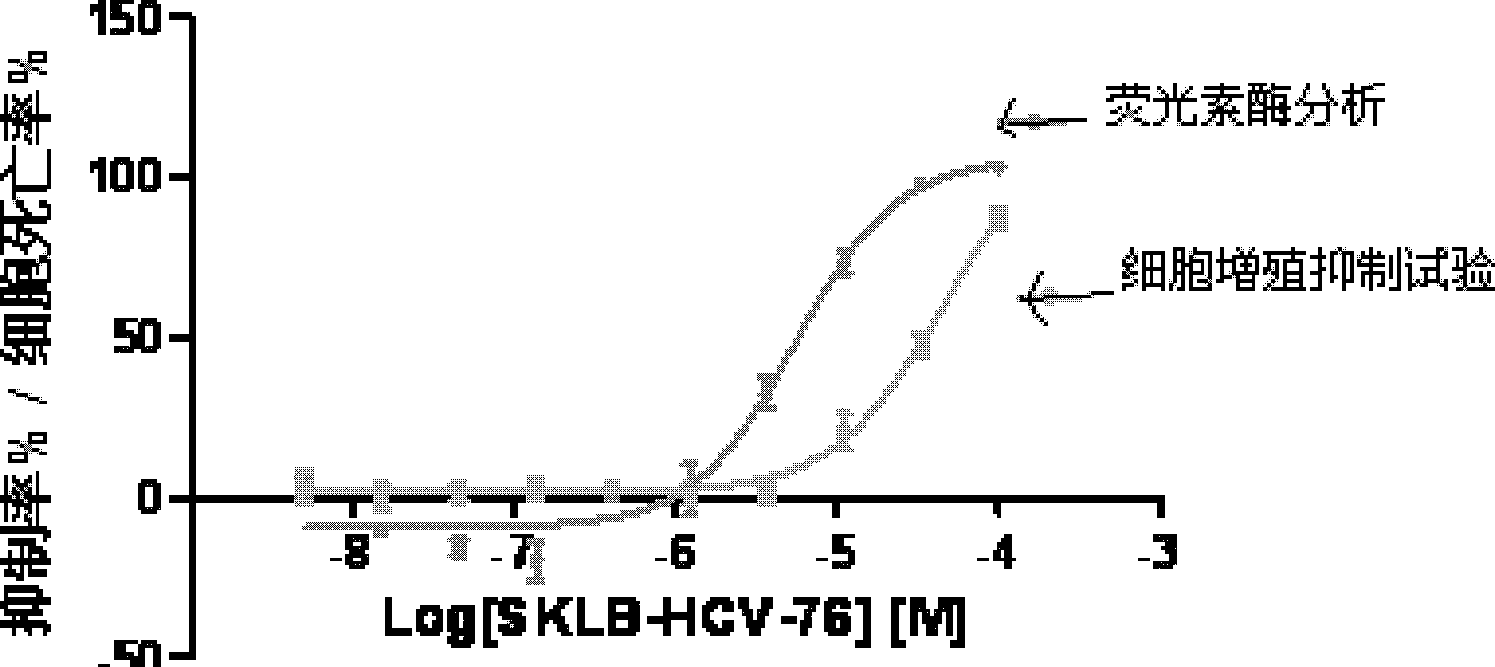
9-sulfonyl-9H-purine derivatives, and preparation method and use thereof
A sulfonyl and derivative technology, applied in the field of chemical medicine, can solve the problems of high treatment cost, serious toxic and side effects, and decreased replication ability of clemizole hydrochloride-resistant virus strains.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The synthesis of embodiment 1 intermediate 6-dimethylaminopurine
[0086]
[0087] Dissolve 0.465g (3mmol) of 6-chloropurine in ethanol, then add 1.25g (9mmol) of potassium carbonate and 0.725g (9mmol) of dimethylamine hydrochloride successively, and then heat to reflux for 12h. After the reaction is completed, the solvent is distilled off under reduced pressure. The remaining solid was suspended in water and stirred for 2 hours, and then filtered to obtain the product 6-dimethylaminopurine with a net weight of 0.392 g and a yield of 80.05%. Purity (HPLC) ≥ 94%
[0088] 1 H-NMR (400MHz, DMSO-D6) δ: 2.47(s, 6H, N(CH 3 ) 2 ), 7.96(s, 1H), 8.38(s, 1H), 13.71(s, 1H).
Embodiment 2
[0089] The synthesis of embodiment 2 intermediate 6-diethylaminopurine
[0090]
[0091] According to the preparation method of the intermediate 6-dimethylaminopurine, the raw materials were replaced with 6-chloropurine and diethylamine, washed and filtered after reaction to directly obtain 6-diethylaminopurine with a yield of 85.37%. Purity (HPLC) > 95%.
[0092] 1 H-NMR (400MHz, DMSO-D6) δ: 1.33(t, 6H, -CH 3 ), 1.33 (m, 2H, -CH 2 -), 7.97(s, 1H), 8.40(s, 1H), 13.66(s, 1H).
Embodiment 3
[0093] The synthesis of embodiment 3 intermediate 6-morpholino purine
[0094]
[0095] According to the preparation method of the intermediate 6-dimethylaminopurine, the raw materials were replaced with 6-chloropurine and morpholine, washed and filtered after reaction to directly obtain 6-morpholinopurine with a yield of 89.42%. Purity (HPLC) > 96%.
[0096] 1 H-NMR (400MHz, DMSO-D6) δ: 3.71(s, 4H, O(CH 2 ) 2 ), 4.21(s, 4H, -N(CH 2 ) 2 ), 8.14(s, 1H), 8.23(s, 1H), 13.07(s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com