Preparation method and quality test method of pseudoephedrine hydrochloride and dextromethorphan hydrobromide chewable tablets
A chewable tablet and safin technology, applied in the field of pharmaceutical preparations, can solve the problems of material loss, unsuitable for chewing tablets, and large dosage, etc., and achieve the effects of improving content uniformity, good bitterness masking effect, and controllable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
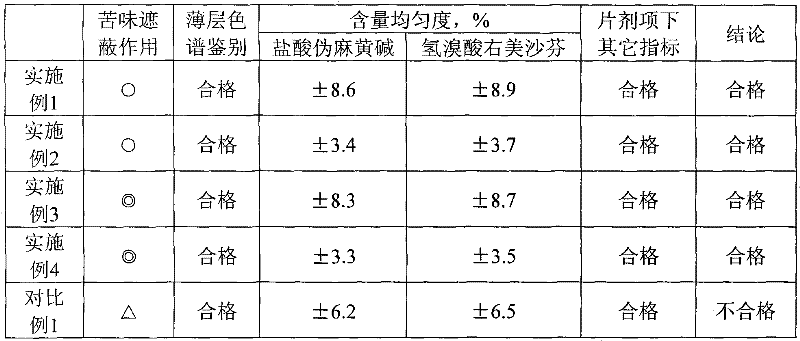
Embodiment 1
[0023] Pseudoephedrine hydrochloride 30g, dextromethorphan hydrobromide 15g, lactose 900g, sodium alginate 2g, sucralose 15g, acrylic resin IV 8g, magnesium stearate 10g, sweet orange essence 2g
[0024] The above-mentioned raw materials are made into 1000 pieces, and the preparation method is as follows:
[0025] Take sodium alginate, add appropriate amount of water to make 2% sodium alginate solution, add pseudoephedrine hydrochloride, dextromethorphan hydrobromide and sucralose, stir to dissolve, then add lactose, stir evenly, granulate, and set aside; take acrylic resin IV, add appropriate amount of ethanol to make a 10% ethanol solution of acrylic resin, add the above granules, stir evenly, granulate, dry, add magnesium stearate and sweet orange essence, mix evenly, and press into tablets to obtain.
Embodiment 2
[0027] Pseudoephedrine hydrochloride 30g, dextromethorphan hydrobromide 15g, lactose 850g, sodium alginate 2g, silicon dioxide 30g, sucralose 15g, acrylic resin IV 8g, magnesium stearate 10g, sweet orange essence 2g
[0028] The above-mentioned raw materials are made into 1000 pieces, and the preparation method is as follows:
[0029] Take sodium alginate, add appropriate amount of water to make 2% sodium alginate solution, add pseudoephedrine hydrochloride, dextromethorphan hydrobromide and sucralose, stir to dissolve, then add silicon dioxide and lactose, stir evenly, granulate, and set aside Take acrylic resin IV, add appropriate amount of ethanol to make a 10% ethanol solution of acrylic resin, add the above granules, stir evenly, granulate, dry, add magnesium stearate and sweet orange essence, mix evenly, and press into tablets to obtain.
Embodiment 3
[0031] Pseudoephedrine hydrochloride 30g, dextromethorphan hydrobromide 15g, lactose 850g, calcium gluconate 20g, sodium alginate 2g, sucralose 15g, acrylic resin IV 8g, magnesium stearate 10g, sweet orange essence 2g
[0032] The above-mentioned raw materials are made into 1000 pieces, and the preparation method is as follows:
[0033] Take sodium alginate, add appropriate amount of water to make 2% sodium alginate solution, add pseudoephedrine hydrochloride, dextromethorphan hydrobromide and sucralose, stir to dissolve, then add lactose and calcium gluconate, stir evenly, granulate, and set aside Take acrylic resin IV, add appropriate amount of ethanol to make a 10% ethanol solution of acrylic resin, add the above granules, stir evenly, granulate, dry, add magnesium stearate and sweet orange essence, mix evenly, and press into tablets to obtain.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap