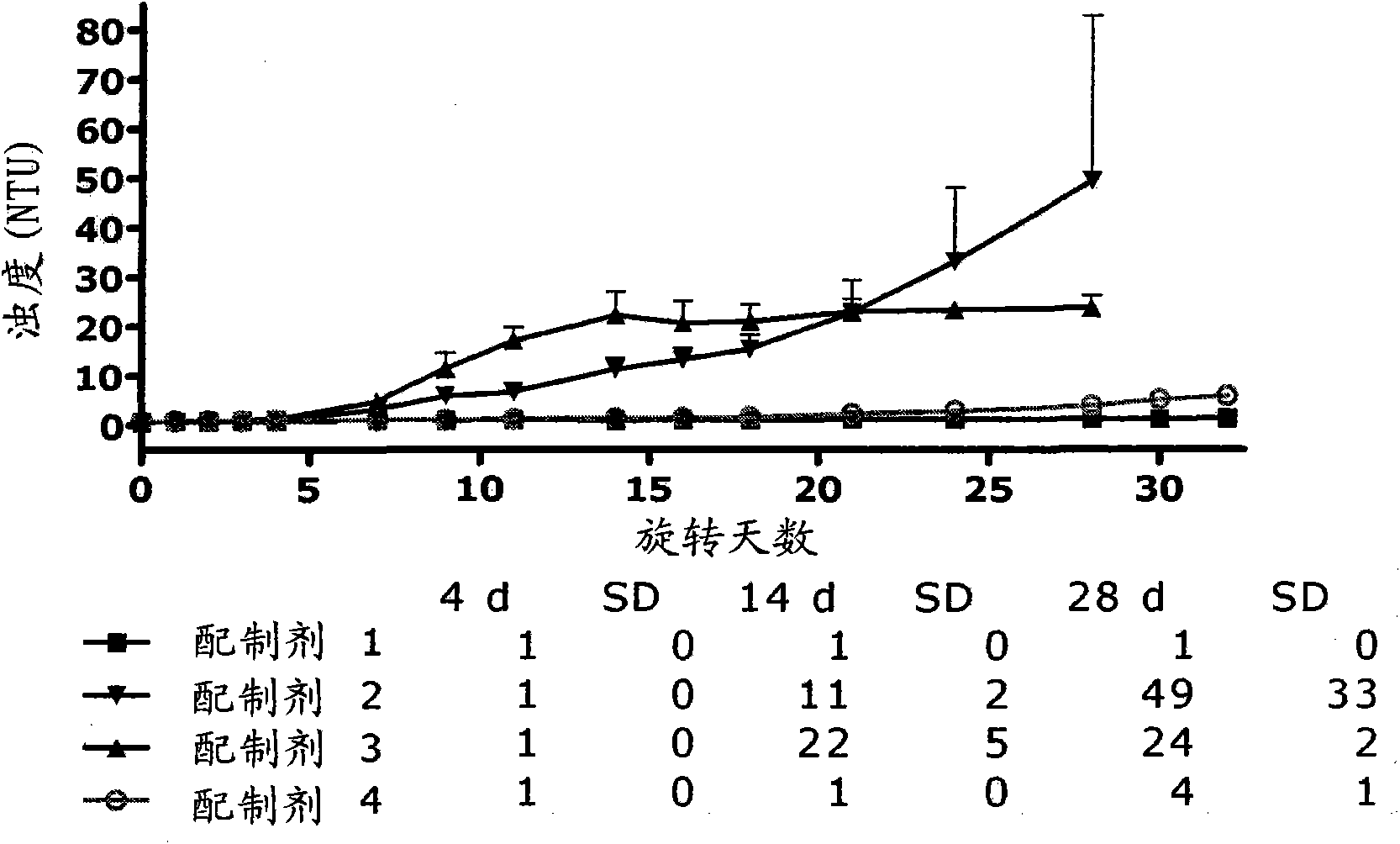
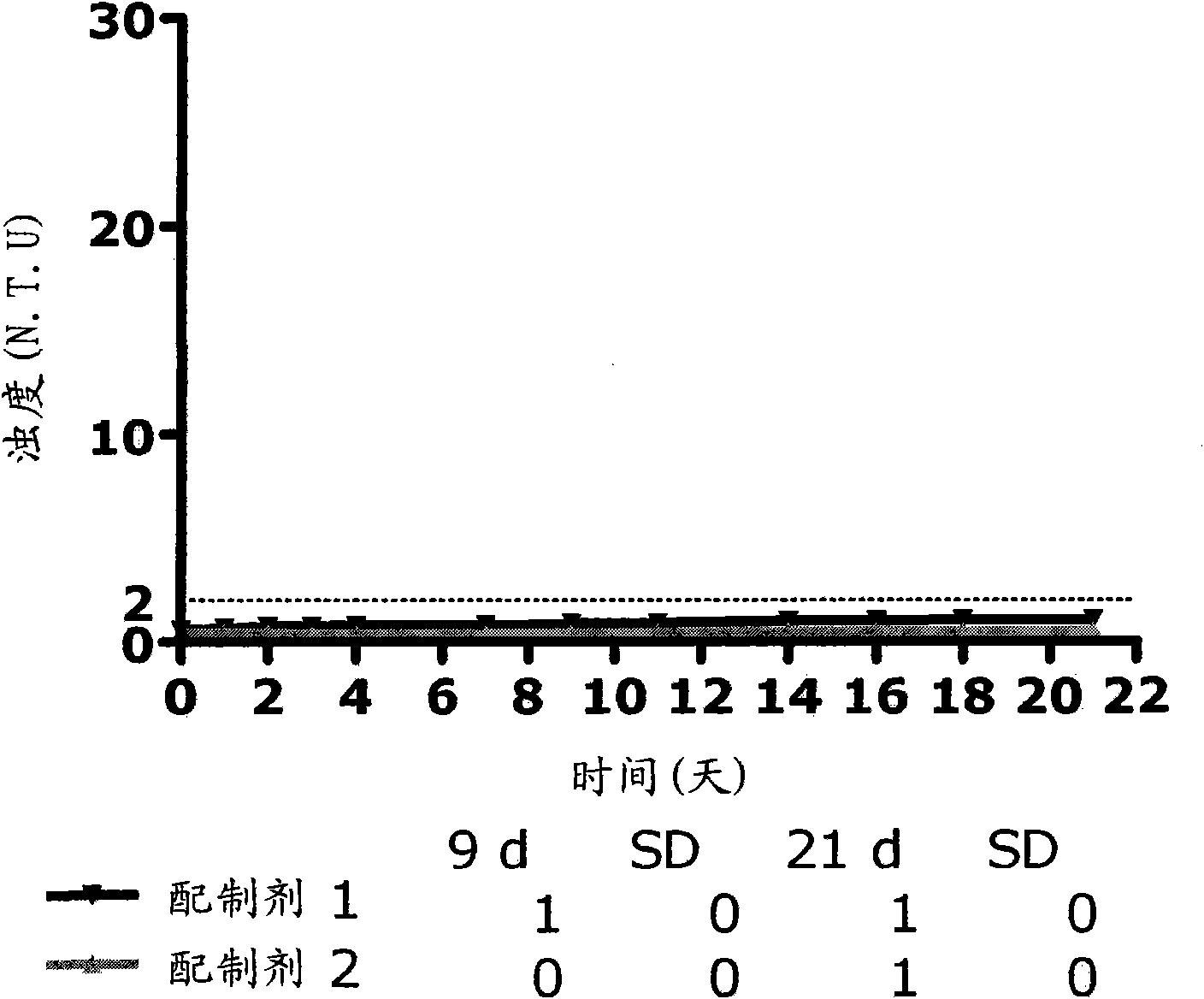
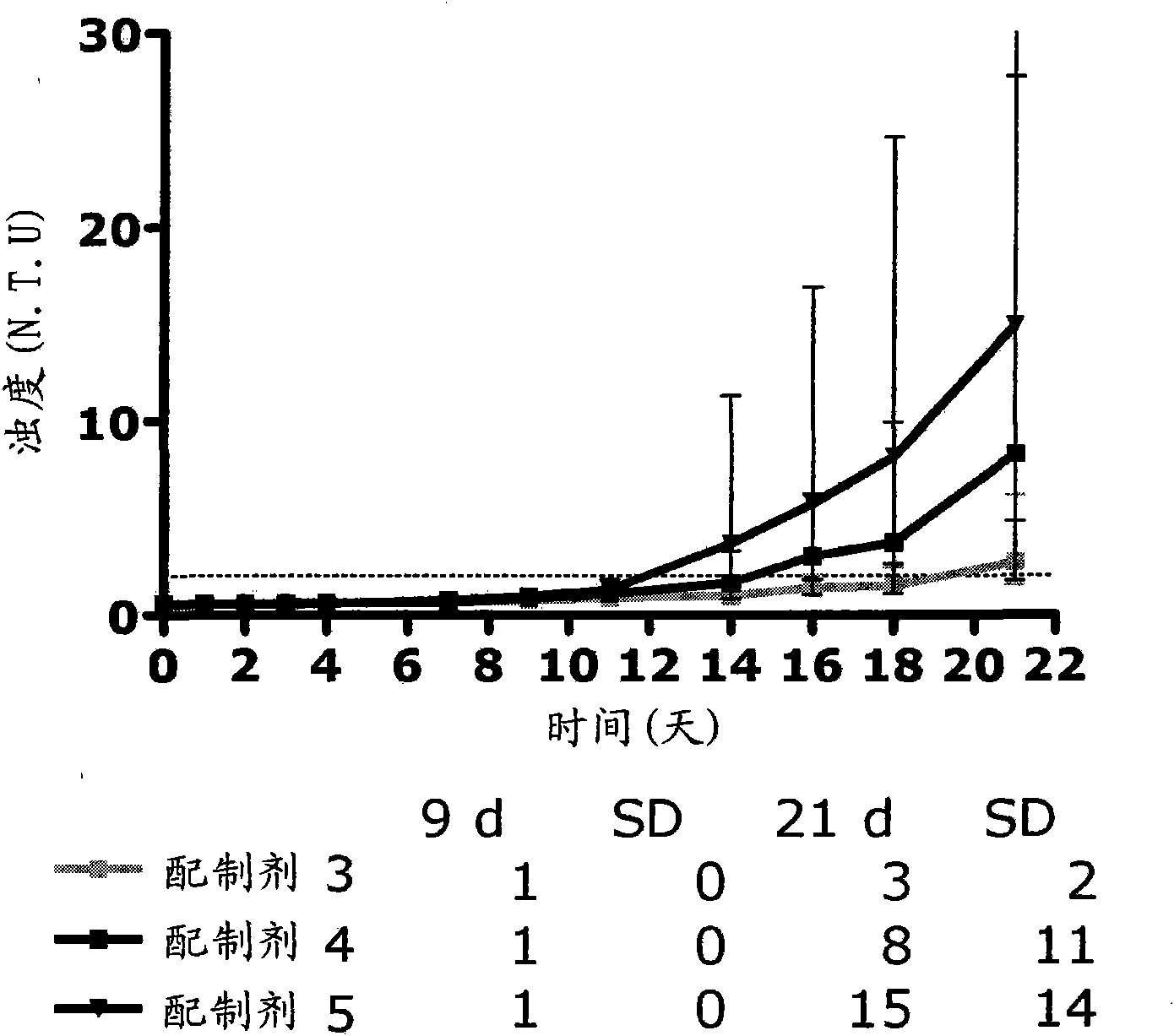
Pharmaceutical compositions comprising GLP-1 peptides or exendin-4 and a basal insulin peptide
A GLP-1, insulin peptide technology, applied in drug combinations, insulin, hormone peptides, etc., can solve problems such as slow onset and prolonged efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0184] Embodiment 1: A soluble pharmaceutical composition for parenteral administration comprising an insulinotropic GLP-1 compound, a basal insulin peptide, a pharmaceutically acceptable additive and zinc, wherein the zinc content is at least 5 Zn ions / 6 insulin molecular.
Embodiment approach 2
[0185] Embodiment 2: A soluble pharmaceutical composition for parenteral administration according to embodiment 1, comprising an insulinotropic GLP-1 compound, a basal insulin peptide, a pharmaceutically acceptable additive and zinc, wherein the zinc content is at least 6 zinc ion / 6 insulin molecules.
Embodiment approach 3
[0186] Embodiment 3: The pharmaceutical composition according to embodiment 1, wherein the zinc content is 5-16 zinc ions / 6 insulin molecules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com