Method for preparing ammonia gas from urea solid by dry pyrolysis
A technology for pyrolysis of gas and urea, which is applied in the fields of climate sustainability, ammonia preparation/separation, sustainable manufacturing/processing, etc. It can solve the problems of large floor space, increased cost, complicated process, etc., and achieve low moisture content. , Small footprint, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
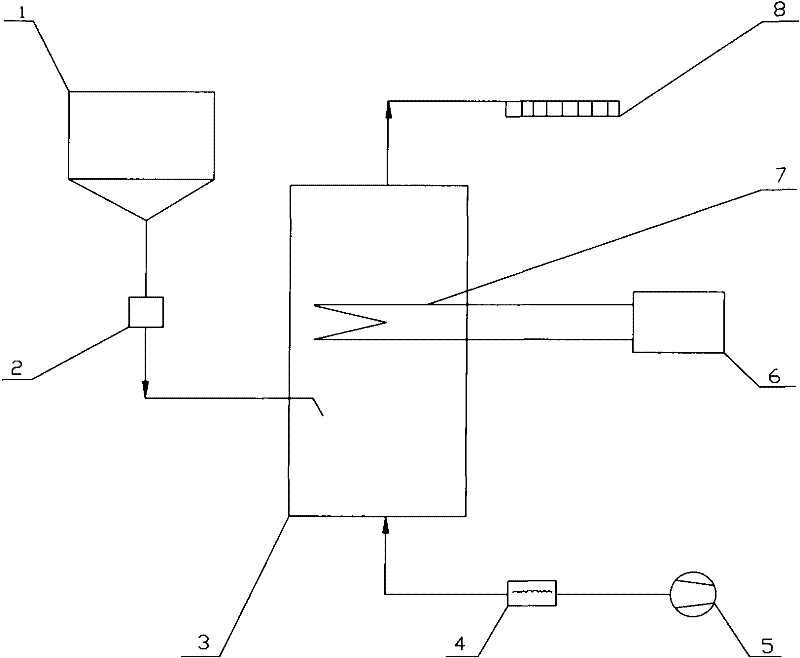
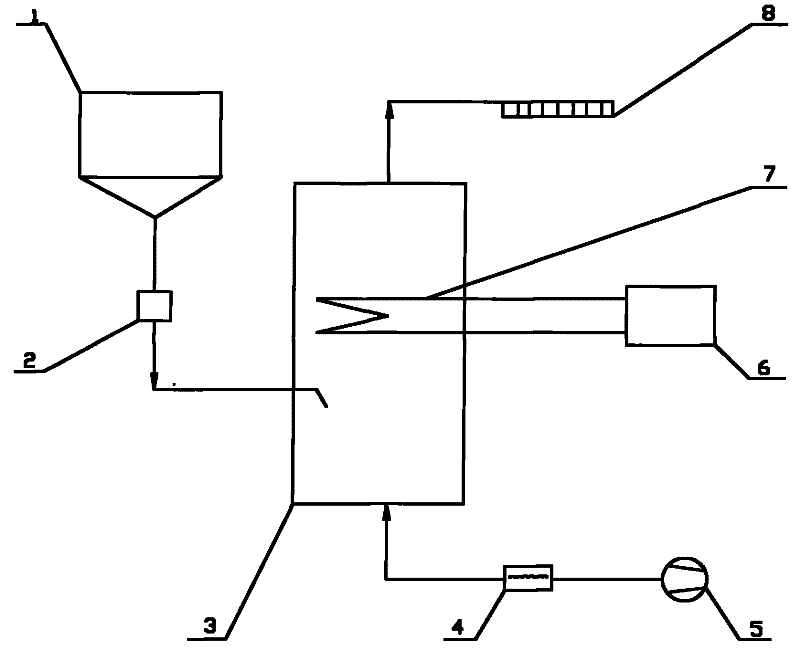
[0030] Below in conjunction with certain 50MW engineering flue gas denitrification project, the present invention is further described:
[0031] Original design data: The flue gas volume of this project is 211600Nm 3 / h, the smoke temperature is 320°C, the water vapor content is 9%, the denitrification efficiency is 48%, the ammonia escape rate is 3ppm, and the urea nitrogen content is 46%.
[0032] (1) The urea solid in the urea solid storage tank 1 is transported into the pyrolysis furnace 3 by the metering and feeding device 2 . The amount of urea added is based on the load signal of the denitrification unit and the NO x Feedback real-time control of the analyzer or CEM system. The calculation formula is:
[0033] G = 0.625 K · Q · C NO × 10 - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com