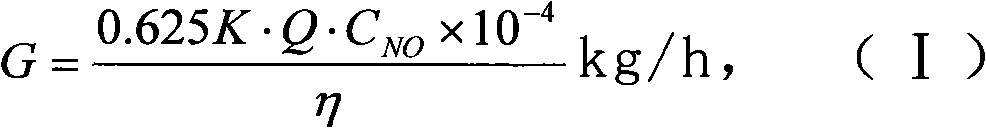Method for preparing ammonia gas from urea solid by dry pyrolysis
A technology of urea and solid, which is applied in the field of urea solid dry pyrolysis to produce ammonia, which can solve the problems of large footprint, high cost, steam consumption, etc., and achieve the effects of low moisture content, small footprint, and reduced equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
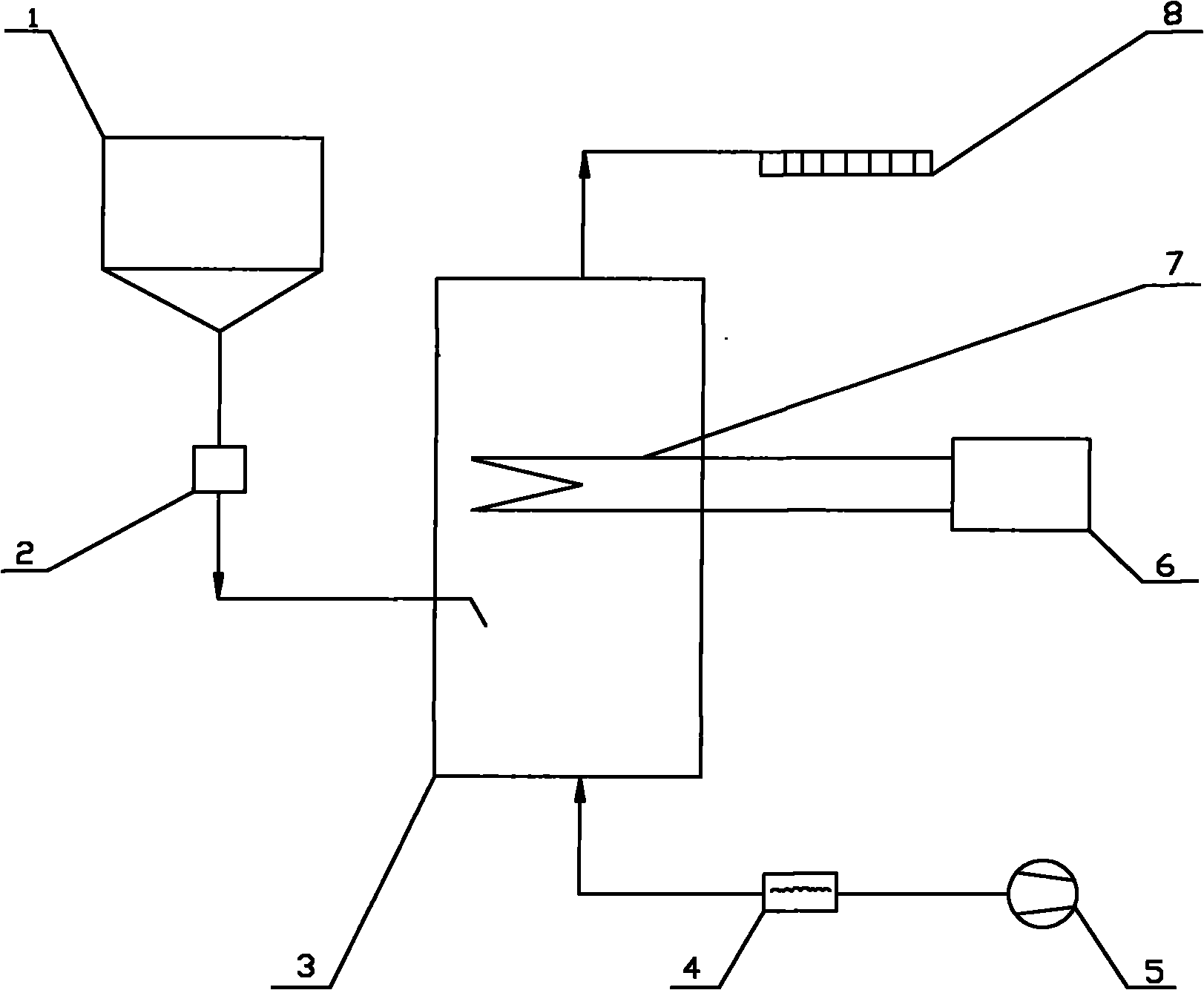
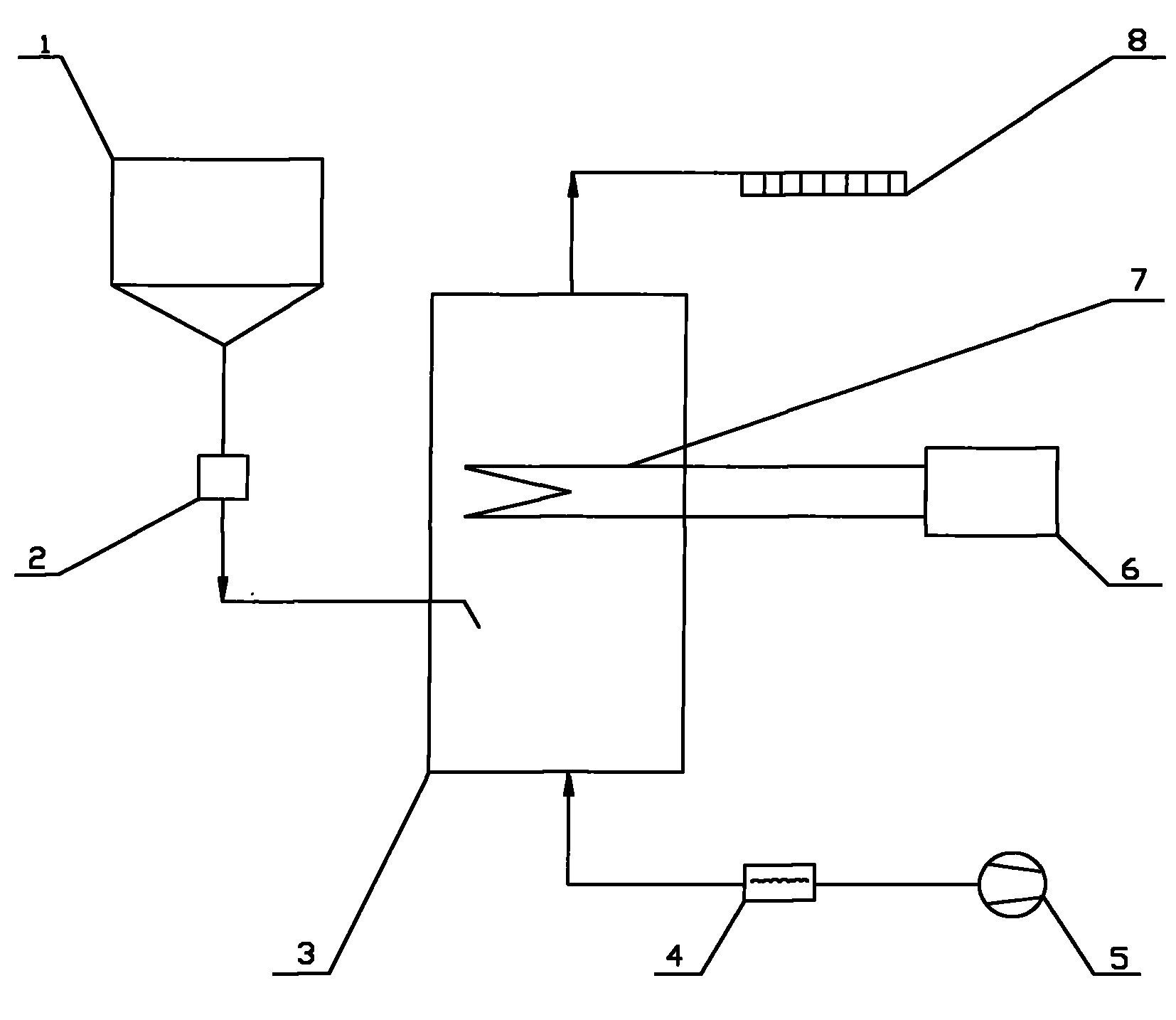
[0030] Below in conjunction with certain 50MW engineering flue gas denitrification project, the present invention is further described:
[0031] Original design data: The flue gas volume of this project is 211600Nm 3 / h, the smoke temperature is 320°C, the water vapor content is 9%, the denitrification efficiency is 48%, the ammonia escape rate is 3ppm, and the urea nitrogen content is 46%.
[0032] (1) The urea solid in the urea solid storage tank 1 is transported into the pyrolysis furnace 3 by the metering and feeding device 2 . The amount of urea added is based on the load signal of the denitrification unit and the NO x Feedback real-time control of the analyzer or CEM system. The calculation formula is:
[0033] G = 0.625 K · Q · C NO × 10 - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com