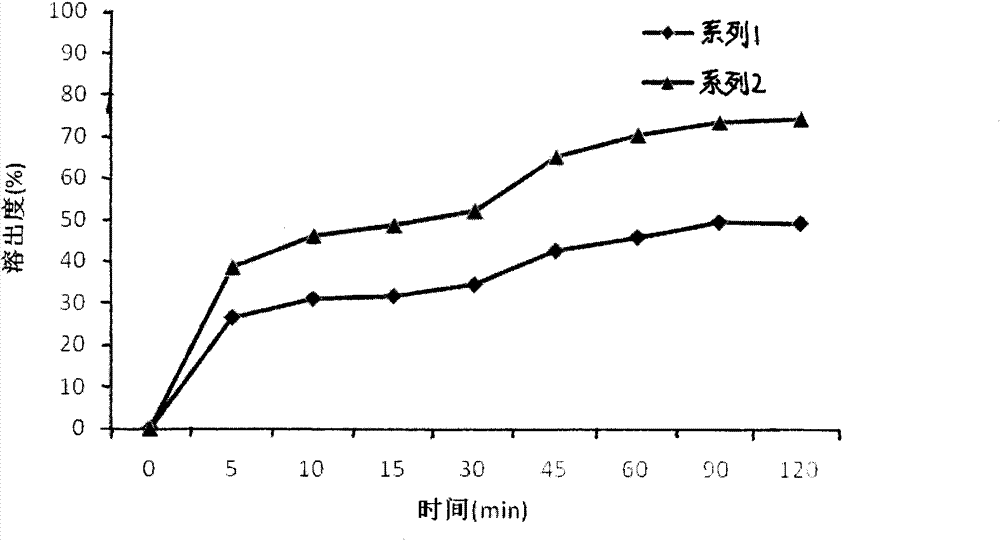
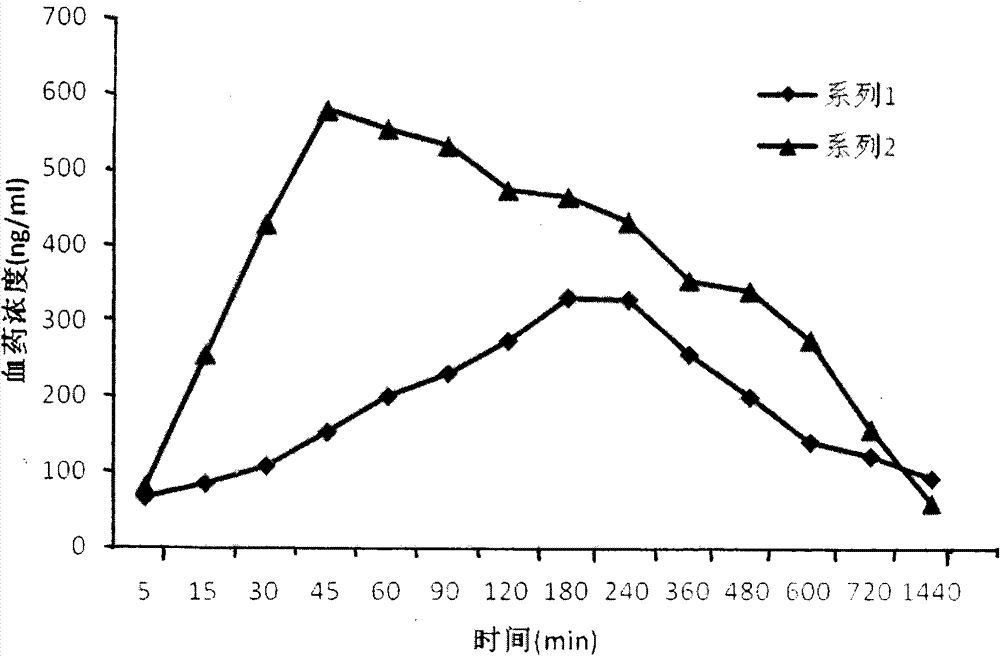
Revaprazan hydrochloride nanosuspension and preparation method thereof
A technology of revaprazan hydrochloride and nano-suspension, which is applied in the field of medicine, can solve the problems of large dosage, poor patient compliance, and low bioavailability, achieve long storage time, uniform dispersion, and solve the problem of insoluble water and The effect of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 1g of revaprazan hydrochloride, dissolve it in 5ml of absolute ethanol, and pour it into an aqueous solution containing 5g of polyethylene glycol 600, 3g of carboxymethylcellulose sodium and 0.002g of propylparaben under stirring, Heat and stir evenly at 60°C and 100 rpm, evaporate the organic solvent to the utmost, and precipitate the drug crystals to obtain revaprazan hydrochloride nanosuspension.
Embodiment 2
[0024] Weigh 1g of revaprazan hydrochloride, dissolve it in 5ml of absolute ethanol, then pour it into an aqueous solution containing 5g of poloxamer 188, 3g of hydroxypropyl cellulose and 0.0015g of propylparaben, and then place Ultrasonic dispersion was carried out in an ultrasonic cleaner for 30 minutes to precipitate the drug crystals to obtain revaprazan hydrochloride nanosuspension.
Embodiment 3
[0026] Weigh 1g of revaprazan hydrochloride, dissolve it in 5ml of absolute ethanol, pour it into an aqueous solution containing 2g of polyvinylpyrrolidone, 3g of sodium carboxymethylcellulose and 0.001g of sorbic acid under stirring, and then use an ultrasonic cell pulverizer Ultrasonic dispersion was performed 30 times (10 s each time, 8 s off) to precipitate the drug crystals to obtain revaprazan hydrochloride nanosuspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com