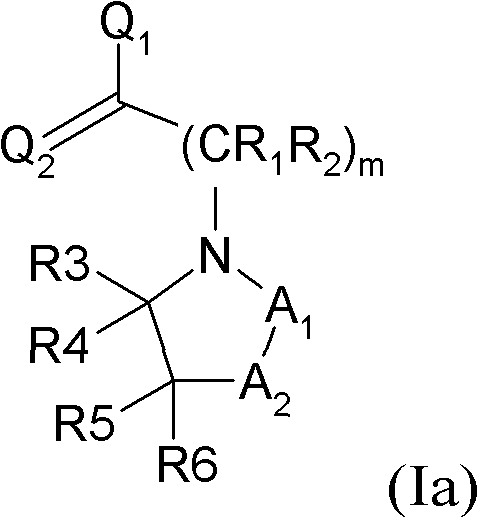
Substituted pyrrolidine and piperidine compounds, derivatives thereof, and methods for treating pain
A compound, chemical formula technology, applied in the field of substituted pyrrolidine and piperidine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0761] Compounds of formula (Ia) and (Ib) were prepared by utilizing the general procedure as shown in Scheme 2 below.
[0762]
[0763] Scenario 2
[0764] To a mixture of pyrrolidine compounds, for example, (S)-(+)-3-fluoropyrrolidine hydrochloride (2.0 g, 15.9 mmol) in dry acetonitrile (30 mL), was added potassium carbonate (4.83 g, 35.0 mmol). The mixture was stirred at room temperature for 5 minutes before adding 2-bromoacetamide (2.08 g, 15.1 mmol). The mixture was stirred overnight at reflux. Strain the hot mixture. The filtrate was recovered, evaporated under reduced pressure and dried in vacuo to provide the described compound.
[0765] Preparation of 2-(1,3-thiazolidin-3-yl)acetamide:
[0766]
[0767] (1.01g, 21%). 1 H NMR (400MHz, DMSO-d6) 2.78(t, J=6.5Hz, 2H), 2.87(s, 2H), 3.00(t, J=6.3Hz, 2H), 7.16(s(br), 1H), 7.35 (s(br), 1H), M+147.
[0768] Preparation of 2-[(3S)-3-fluoropyrrolidin-1-yl]acetamide:
[0769]
[0770] (1.91g, 87%). 1 H NMR (400...
Embodiment 2
[0822] Example 2: Analgesic effect of compounds of the present invention
[0823] Determination of analgesic effects in experimental models of neuropathic pain
[0824] Using the procedure described hereafter, it was determined that 2-(pyrrolidin-1-yl)acetamide, 3-(piperidin-1-yl)propionamide, 3-[(3S)-3-fluoropyrrolidin-1-yl ] propionamide, and the analgesic effect of 3-[(3R)-3-(dimethylamino)pyrrolidin-1-yl] propionamide. Other methods are known to the skilled person.
[0825] Adult, male St.-Dow rats were obtained from Charles River Laboratories (St Constant, QC) and housed under standard conditions at the Institut Armand Frappier (Laval, QC). Experimental animals were provided food and water ad libitum and rats weighed 175-200 grams at the time of evaluation.
[0826] Compounds for intrathecal administration were prepared by dissolving the compounds in a vehicle of D5W (5% glucose); the total volume of solution administered to rats was 20 ul, and the amount of exemplary ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com