Method for producing cryolite by using fluorine-containing waste residues of electrolytic aluminium
A technology of electrolytic aluminum and cryolite, applied in the field of aluminum metal smelting, can solve problems such as environmental pollution, failure to return electrolytic aluminum, waste of fluorine resources, etc., achieve significant economic benefits, low production costs, and solve the bottleneck of development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
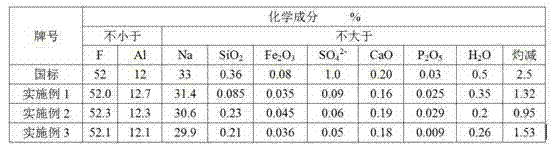
Embodiment 1
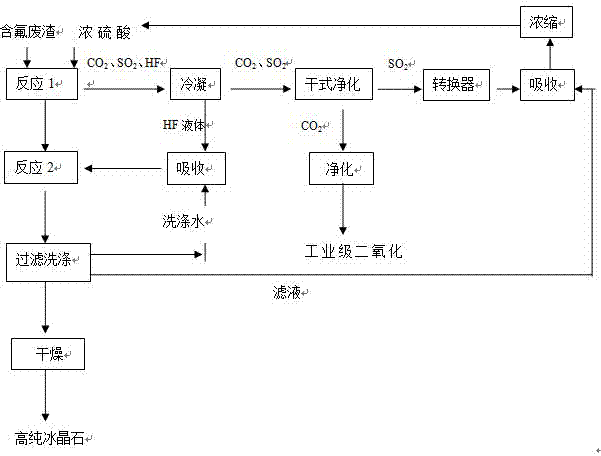
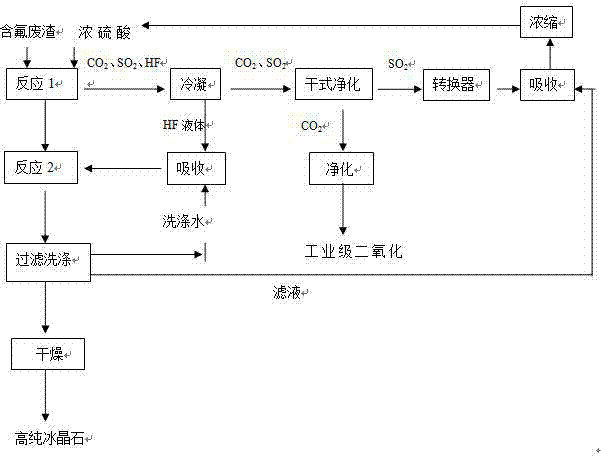
[0024] The method that present embodiment utilizes the fluorine-containing waste residue of concentrated sulfuric acid and electrolytic aluminum to produce cryolite comprises the following steps:
[0025] (1) React fluorine-containing waste residue with 98% concentrated sulfuric acid four times its mass at a temperature of 450°C for 5 hours;
[0026] (2) The sulfur dioxide, carbon dioxide, and hydrogen fluoride gases generated during the reaction are condensed by the condenser (the boiling point of HF is 19.54°C);
[0027] (3) After the condensation is completed, the hydrogen fluoride liquid in the lower part of the condenser is absorbed by adding water to a concentration of 40% for subsequent reactions;
[0028] (4) Sulfur dioxide and carbon dioxide gas from the upper part of the condenser are separated from 90% of carbon dioxide by the dry purification equipment cyclone dust collector;
[0029] (5) Purify the sulfur dioxide gas from which carbon dioxide has been separated t...
Embodiment 2
[0034] The method that present embodiment utilizes the fluorine-containing waste residue of concentrated sulfuric acid and electrolytic aluminum to produce cryolite comprises the following steps:
[0035] (1) React fluorine-containing waste residue with 98% concentrated sulfuric acid at 250°C for 8 hours at 250°C;
[0036] (2) The sulfur dioxide, carbon dioxide, and hydrogen fluoride gases generated during the reaction are condensed by the condenser (the boiling point of HF is 19.54°C);
[0037] (3) After the condensation is completed, the hydrogen fluoride liquid in the lower part of the condenser is absorbed with washing water to a concentration of 30% for subsequent reactions;
[0038] (4) Sulfur dioxide and carbon dioxide gas from the upper part of the condenser are separated from 90% of carbon dioxide by the electrostatic precipitator of the dry purification equipment;
[0039] (5) Purify the sulfur dioxide gas from which carbon dioxide has been separated through a wet p...
Embodiment 3
[0044] The method that present embodiment utilizes the fluorine-containing waste residue of concentrated sulfuric acid and electrolytic aluminum to produce cryolite comprises the following steps:
[0045] (1) React fluorine-containing waste residue with concentrated sulfuric acid with a concentration twice the mass of 98% at a temperature of 150°C for 12 hours;
[0046] (2) The sulfur dioxide, carbon dioxide, and hydrogen fluoride gases generated during the reaction are condensed by the condenser (the boiling point of HF is 19.54°C);
[0047] (3) After the condensation is completed, the hydrogen fluoride liquid in the lower part of the condenser is absorbed with washing water to a concentration of 25% for subsequent reactions;
[0048] (4) Sulfur dioxide and carbon dioxide gas from the upper part of the condenser are separated from 90% of carbon dioxide by the dry purification equipment cyclone dust collector;
[0049] (5) Purify the sulfur dioxide gas from which carbon dioxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com