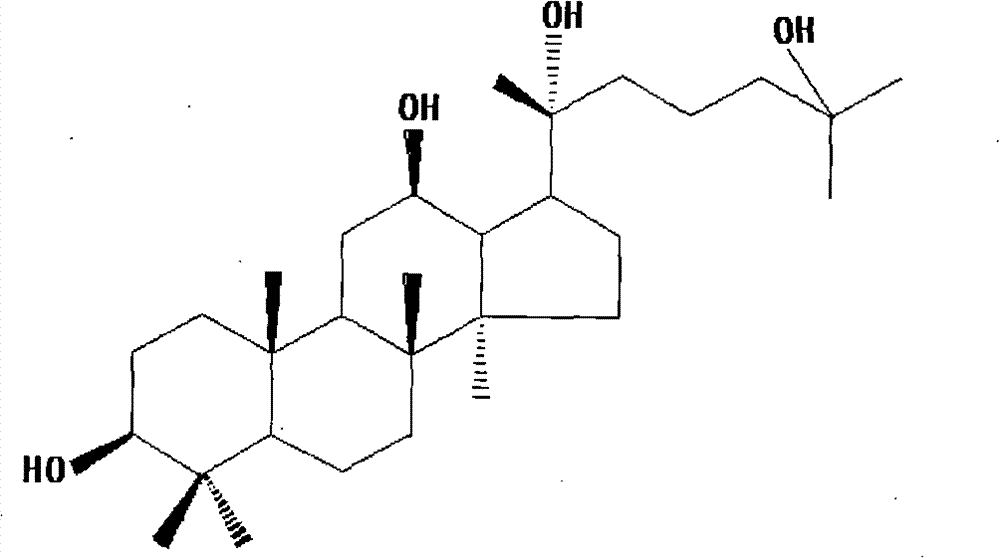
Method for preparing 20(R)-25-hydroxy-dammarane type-3beta,12beta,20-triols
A technology of dammarane type and hydroxyl group, which is applied in the field of preparation of 20(R)-25-hydroxy-dammarane type-3β,12β,20-triol, and can solve problems such as difficulty in obtaining a large amount of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of 20(R)-25-OH-PPD by silica gel chromatography
[0019] 5kg dried Araliaceae ginseng fresh ginseng fruit was extracted with 70% ethanol, passed through a D101 macroporous adsorption resin column (provided by Tianjin Chemical Co., Ltd.), washed with water and eluted from the column with 70% ethanol to separate and purify it , After drying, the total saponins of ginseng fruit are obtained. Take 10 g of total saponins from ginseng fruit, extract with chloroform, and separate the chloroform extract by silica gel column chromatography. 7 fractions are obtained by gradient elution of chloroform: methanol (30: 1-5: 1), and fraction 5 is passed through a reverse-phase silica gel column layer. 20(R)-25-OH-PPD was obtained after separation by precipitation, elution with acetonitrile: water (80:20), and recrystallization from ethyl acetate in a yield of 0.18%.
Embodiment 2
[0020] Example 2: 20(R)-25-OH-PPD was prepared by alkaline hydrolysis
[0021] 10 g of total ginsenosides were weighed, dissolved in 1000 ml of 95% ethanol solution, filtered to remove insoluble matter to obtain a filtrate. The prepared 0.5% (W / V) sodium hydroxide ethanol solution was added to the above-mentioned filtrate under stirring, kept at rest, filtered, washed with water and precipitated to neutrality. The dried precipitate was separated by silica gel column chromatography, and 8 fractions were obtained by gradient elution of petroleum:ethyl acetate (30:1-1:1). Fraction 5 was recrystallized from ethyl acetate to give 20(R) -25-OH-PPD white crystal, yield 1.2%.
Embodiment 3
[0022] Example 3: Preparation of 20(R)-25-OH-PPD by Sodium Hydroxide Hydrolysis
[0023] Weigh 10 g of total saponins from ginseng fruit, dissolve it in 1000 ml of methanol aqueous solution with a sodium hydroxide concentration of 2.5 mol / L and a concentration of 80% for 24 h, neutralize the reaction solution with 2.5 mol / L hydrochloric acid, recover methanol under reduced pressure, and extract with chloroform The reaction solution, the chloroform phase was washed with water, dried over anhydrous sodium sulfate, evaporated to dryness to collect the residue, separated by silica gel column chromatography, and eluted with a petroleum:ethyl acetate (50:1-2:1) gradient to obtain 8 fractions, Fraction 5 was recrystallized from ethyl acetate to obtain 20(R)-25-OH-PPD white crystals in a yield of 1.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com