Quadruple inactivated vaccine for preventing chicken diseases
A technology for inactivating vaccines and diseases, applied to medical preparations containing active ingredients, antibody medical ingredients, pharmaceutical formulas, etc., can solve the problems of high variability of avian influenza virus, economic losses of farmers, and increased difficulty of prevention and control , to achieve the effect of reducing immunization cost and immune stress response, normal eggshell quality and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: poison seed
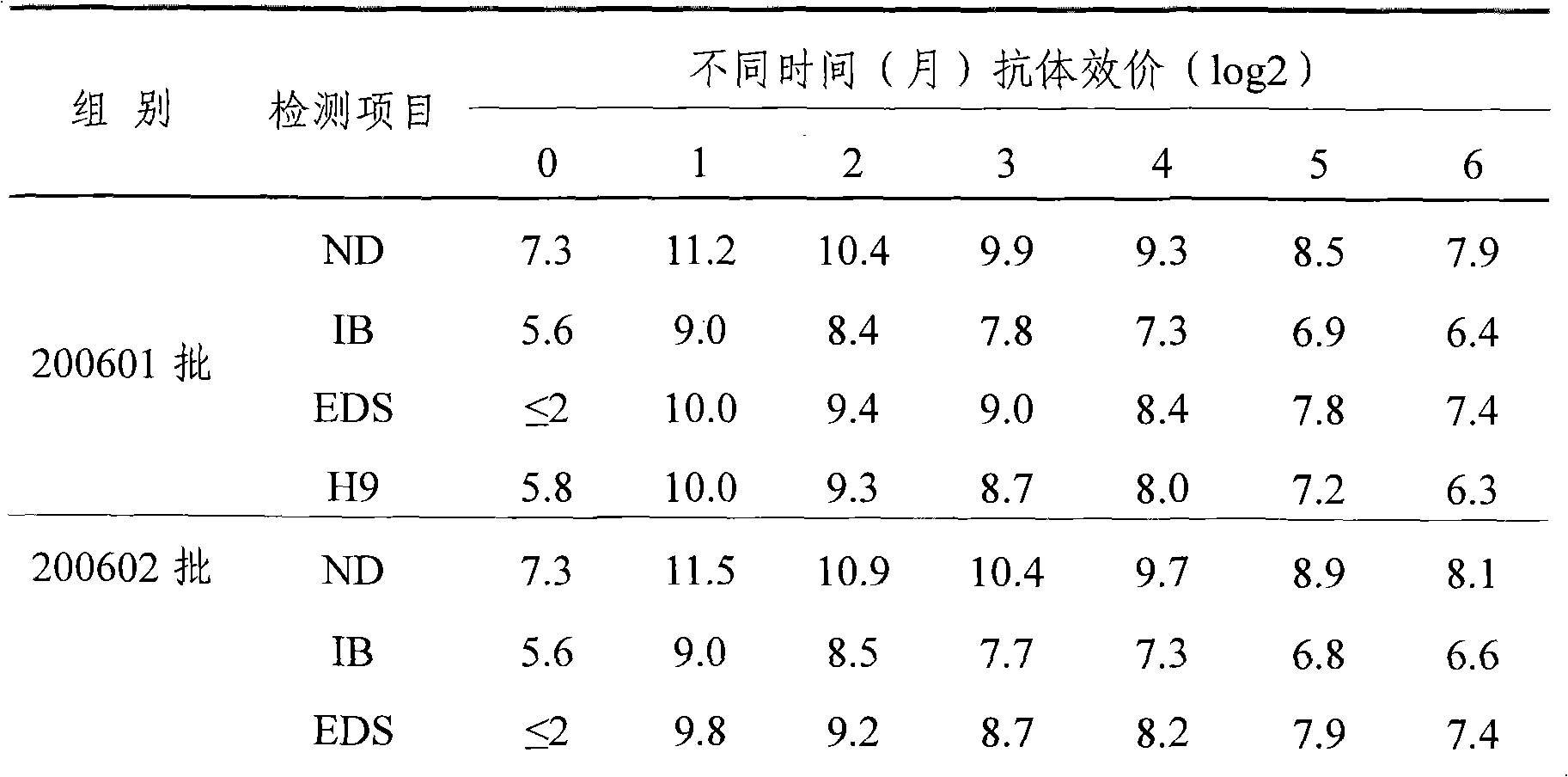
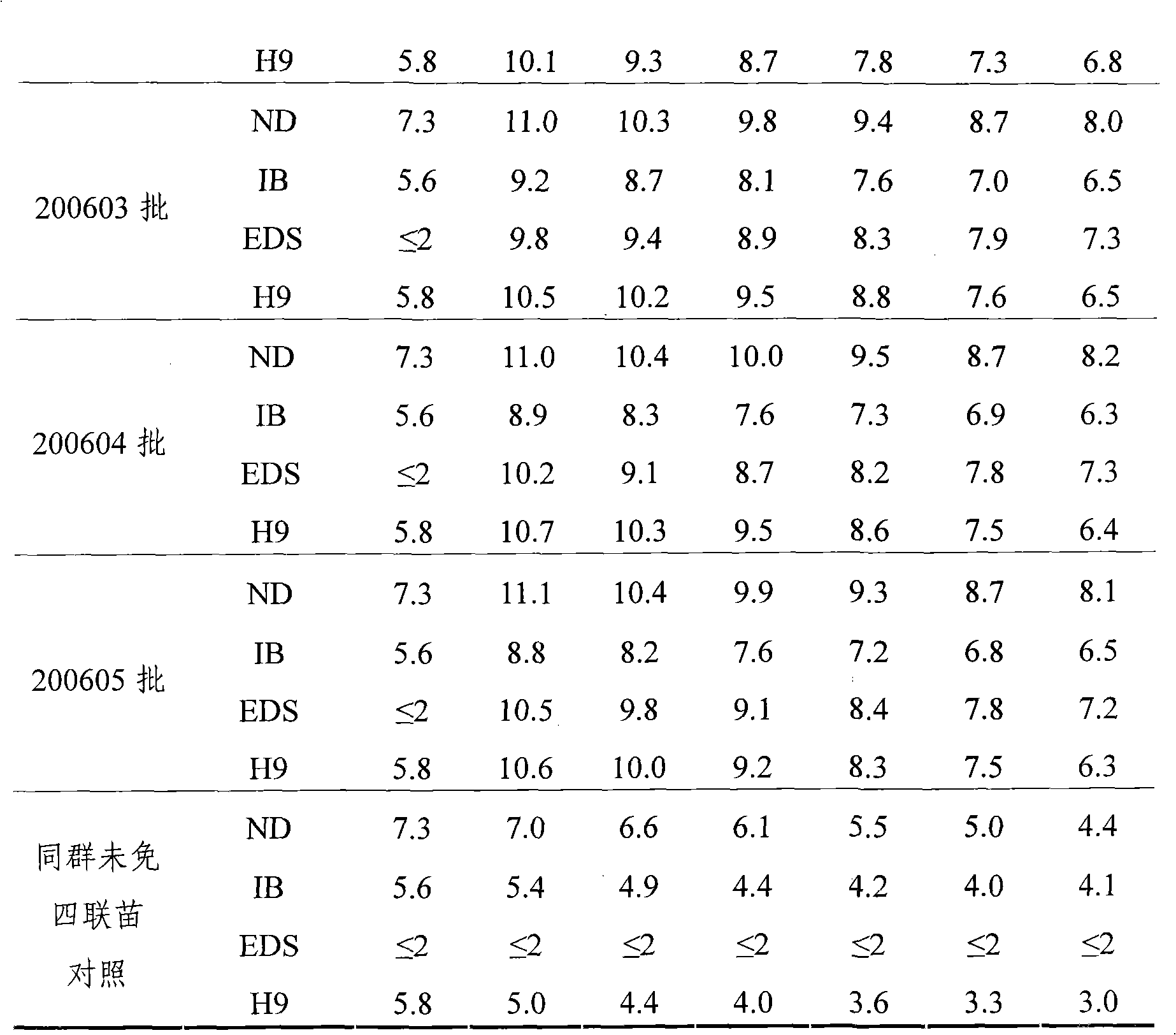
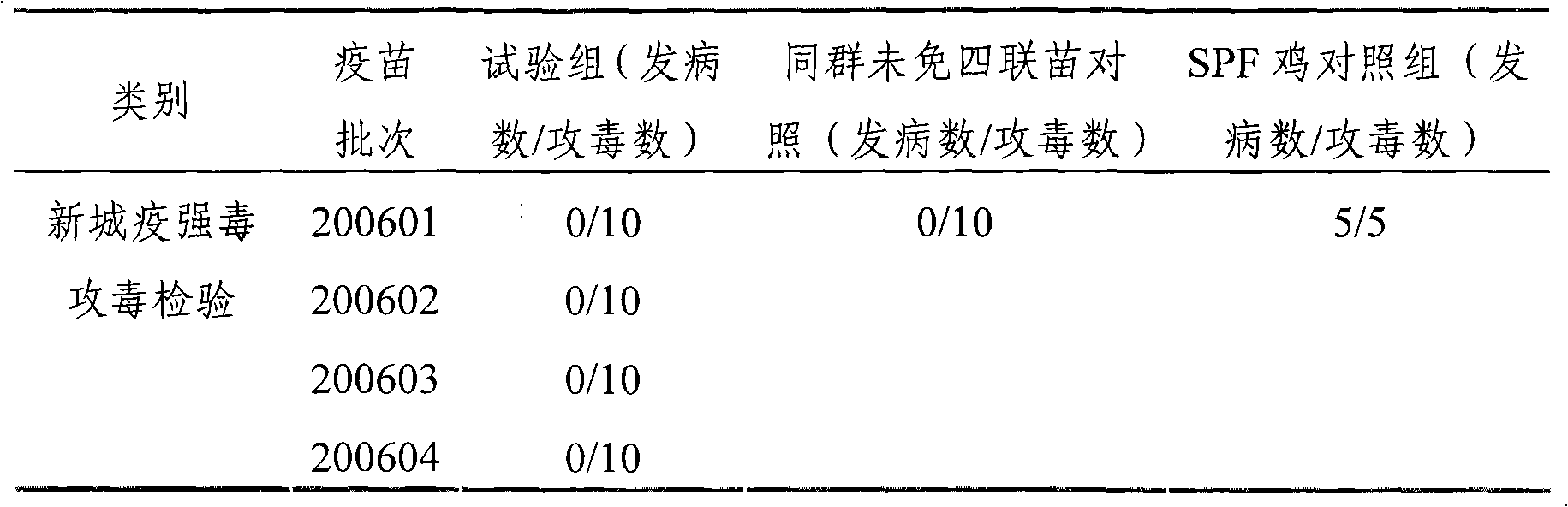
[0029] 1.1 Virus seed source The chicken Newcastle disease virus seed that makes this product is chicken Newcastle disease virus ZM10 strain, and the virus strain used for effect inspection is chicken Newcastle disease virus virulent Beijing strain (CVCC AV1611 strain), and the chicken infectious bronchitis virus virus seed used for seedling making and effect inspection is M41 strains are all identified, kept and supplied by the China Veterinary Drug Administration; the egg drop syndrome virus virus used for seedling production and efficacy testing is EDS 76 - AV127 strain, the avian influenza virus seed used for seedling preparation and effect test is A / Chicken / Shandong / S2 / 1999 (H9N2) strain (S2 strain for short), all identified and kept by the Poultry Research Institute of Shandong Academy of Agricultural Sciences.
[0030] 1.2 Poison Seed Standards
[0031] 1.2.1 Newcastle disease virus ZM10 strain should meet the following standards
[...
Embodiment 2
[0074] Embodiment 2: Preparation of poisonous seeds for production
[0075] Dilute chicken Newcastle disease virus ZM10 strain, chicken infectious bronchitis M41 strain and avian influenza virus H9 subtype S2 strain respectively with sterilized normal saline and inoculate 9-11-day-old SPF chicken embryos in the allantoic cavity, 36 Incubate at ~37°C for 48-96 hours, then cool at 2-8°C for 12-24 hours; then aseptically harvest chicken embryo allantoic fluid, and take chicken embryo fluid that is sterile and has an agglutination value of ≥9log2 for 1% chicken erythrocytes Mixed as the virus species for the production of chicken Newcastle disease virus ZM10 strain, tested for sterility and virus content ≥ 10 6.0 EID 50 / 0.1ml, the chicken embryo fluid that is negative for 1% chicken erythrocyte agglutination test is mixed as the virus seed for the production of chicken infectious bronchitis M41 strain, and the chicken embryo fluid that is tested for sterility and has an agglutin...
Embodiment 3
[0077] Embodiment 3: Vaccine manufacturing production process
[0078] 1. The impact of different dilutions of virus species used in production on virus content in chicken embryos
[0079] Carried out the impact experiment of different dilution multiples of virus species on the virus content in the harvest chicken embryo liquid, through repeatedly repeating, the result confirms: ZM10 strain 10000 times and 1000 times of dilutions are inoculated with SPF chicken embryos, and the amount of virus harvested is consistent (10 8.83 EID 50 / 0.1ml); AV-127 strain 1000 times diluted inoculated susceptible duck embryos, the virus content was consistent with 100 times the amount of virus harvested (107.38EID50 / 0.1ml); and M41 strain 100 times diluted inoculated SPF chicken embryos, the virus of harvested Quantity (10 7.16 EID 50 / 0.1ml), it is significantly higher than the 1000-fold dilution (10 6.16 EID 50 / 0.1ml); S2 strain 1000-fold dilution was inoculated with SPF chicken embryo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com