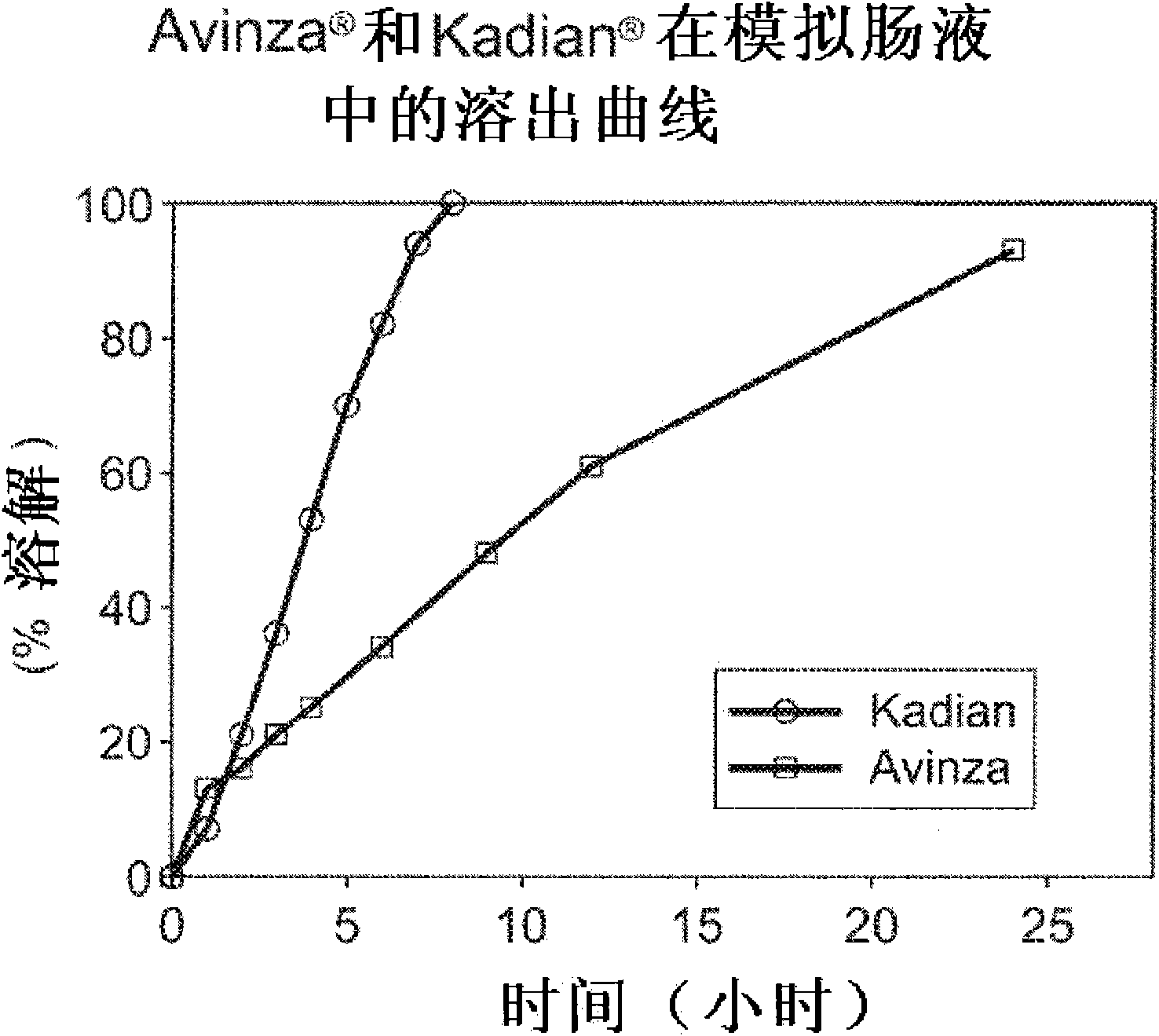
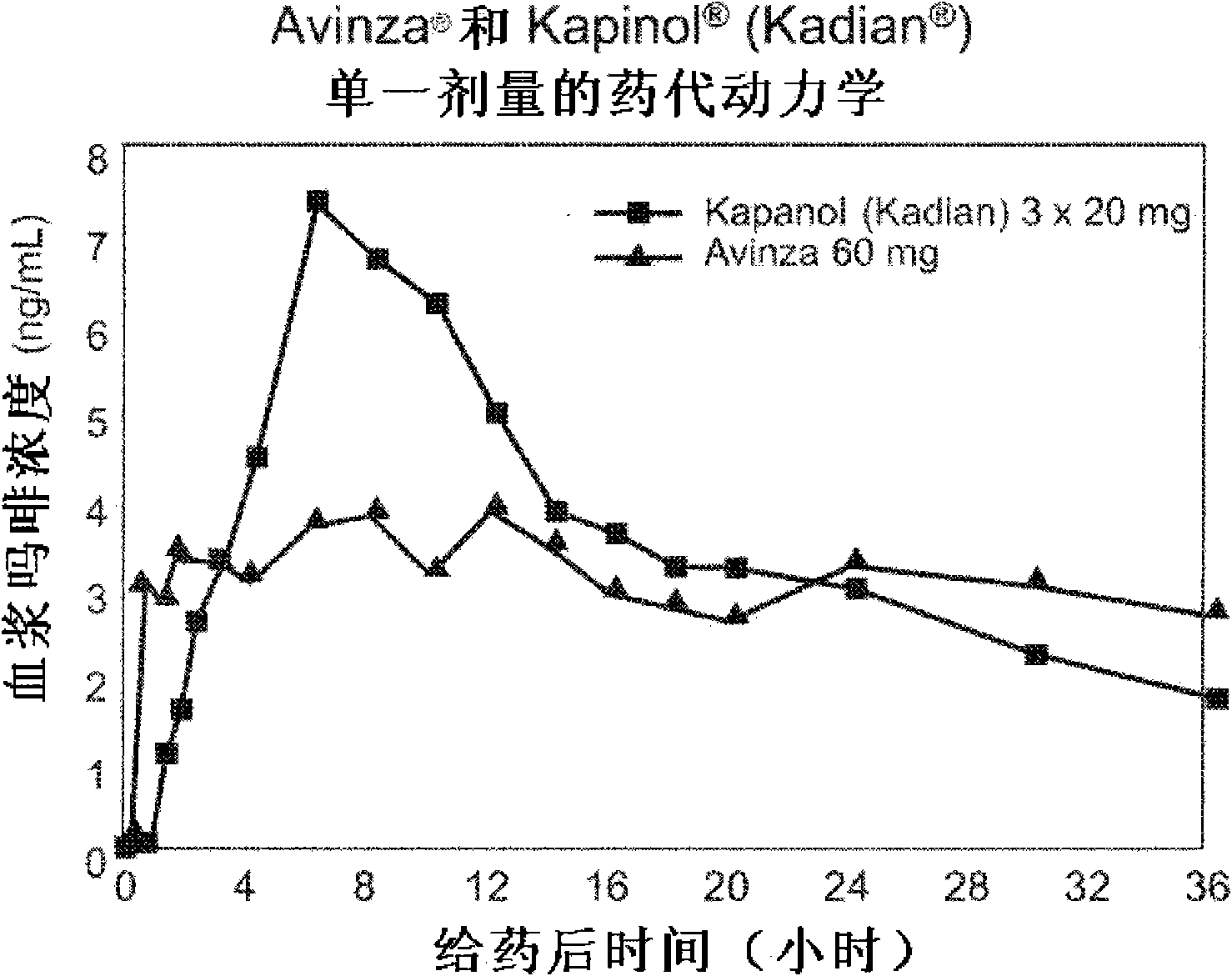
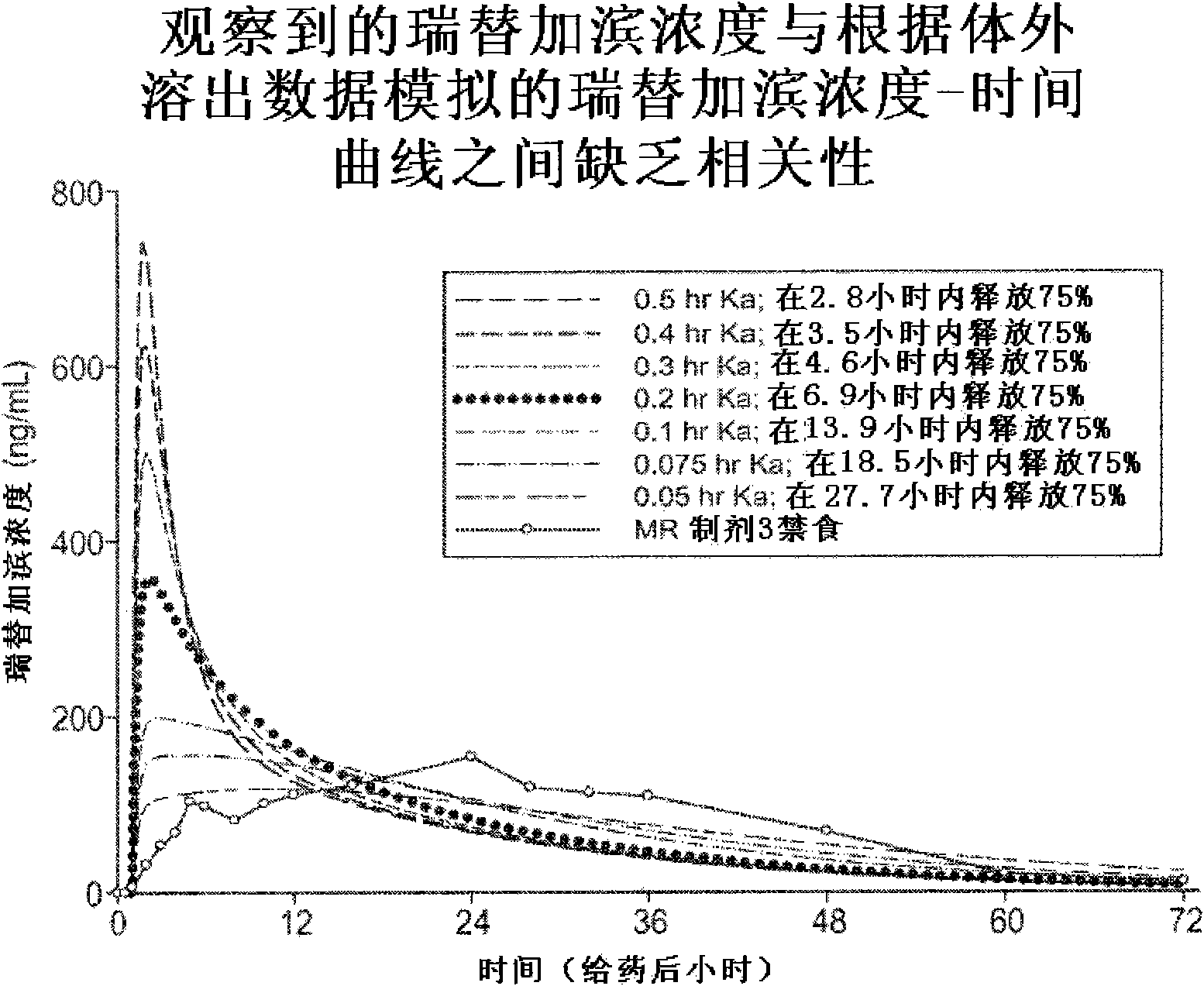
Modified release formulation and methods of use
一种调控释放、制剂的技术,应用在有效治疗神经系统过度兴奋的药物制剂领域,能够解决浓度水平波动等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0086] Composition and ratio of modified release formulations
[0087] This example illustrates the composition and composition ratio of the formulation of the compound of formula I.
[0088] Table 1 provides the ingredients and their ratios used to formulate the pharmaceutical composition into a modified release dosage form. For all the following examples, the proportion of active ingredients used in the total dosage form varies from 35% to 65%, and the proportions of the remaining binders, disintegrants, surfactants, release modifiers, glidants or lubricants are shown in the table 1 range shown. Dry blending for direct compression or wet granulation of a portion of the formulation or wet granulation of the entire formulation can be used to produce granules and tablets.
[0089] Table 1. Exemplary Modified Release (MR) Formulations of Retigabine
[0090]
Embodiment II
[0092] Preparation of Modified Release Formulations
[0093] This example illustrates the method for preparing the modified release formulation of the present invention, and provides the constituent components and their respective ratios for formulating the modified release formulation of the present invention.
[0094] The methods described herein will be understood by those skilled in the art, since many such methods are well known in the art. Table 2 shows the ingredients and ratios used in the formulation of several embodiments of the claimed invention. It will be appreciated that the amounts and ratios of the constituents used in Tables 1 and 2 may be subdivided into smaller or larger amounts while maintaining the compositional ratios to produce different modified release formulations of the invention. It should be further understood that such ratios of constituents are also within the scope of the claims and the scope of the present invention.
[0095] Modified relea...
Embodiment III
[0108] Preparation of Modified Release Formulations Containing Different Amounts of Active Ingredient
[0109] This example describes the composition and ratios of several modified release formulations of the invention containing 200 mg retigabine.
[0110] Several modified release formulations were prepared with 200 mg retigabine and different proportions of the components of the present invention. Table 4 provides several modified release formulations containing 200 mg retigabine. In parentheses are the composition ratios per milligram tablet. For Formulation 9, additional granular SDS was used to prepare the composition. It will be appreciated that one skilled in the art can use larger or smaller partitioned components, as described in Table 4, while maintaining the ratios of the components, to produce similar modified release formulations. It is also understood that such allocations are within the scope of the present invention.
[0111] Modified release formulations...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com