Patents
Literature
33 results about "Carbamic acid ethyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for improving the water transport characteristics of hydrophobic surfaces
ActiveUS20080172937A1Easy to transportLow washout rateSeed and root treatmentCultivating equipmentsArylEther
The invention provides compounds having the general structure:wherein R and R′ are independently selected from the group consisting of H, C1-24 alkyl, aryl, C1-24 alkylaryl, aryl(C1-24)alkyl, —C(═O)—R1 (esters), C(═O)—NHR1 (urethanes), or C(═O)—O—R1 (carbonates) wherein R1 is selected from the group consisting of C1-24 alkyl, aryl, C1-24 alkylaryl, C1-24 arylalkyl; A is an organic moiety derived from the group consisting of alkylene oxides having 4-12 carbon atoms and aryl epoxides having 8-12 carbon atoms; x=1-300; y=0-200; z=0-200; and with the proviso that R and R′ can not be H or ether functionality at the same time. The compounds are useful for improving the water transport characteristics of hydrophobic surfaces.
Owner:ETHOX CHEM LLC
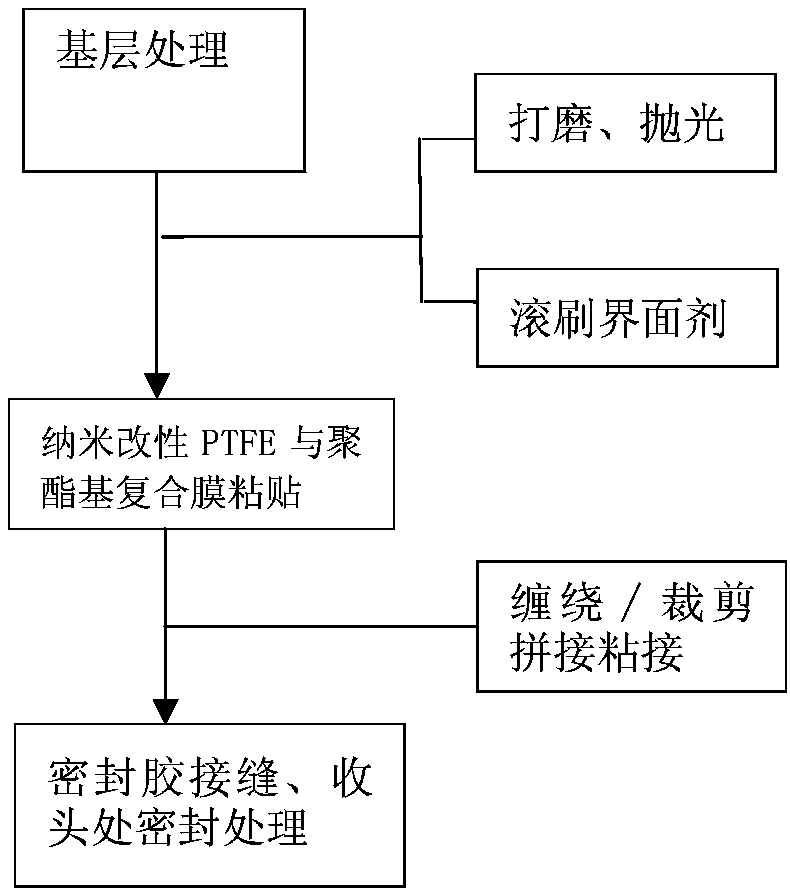
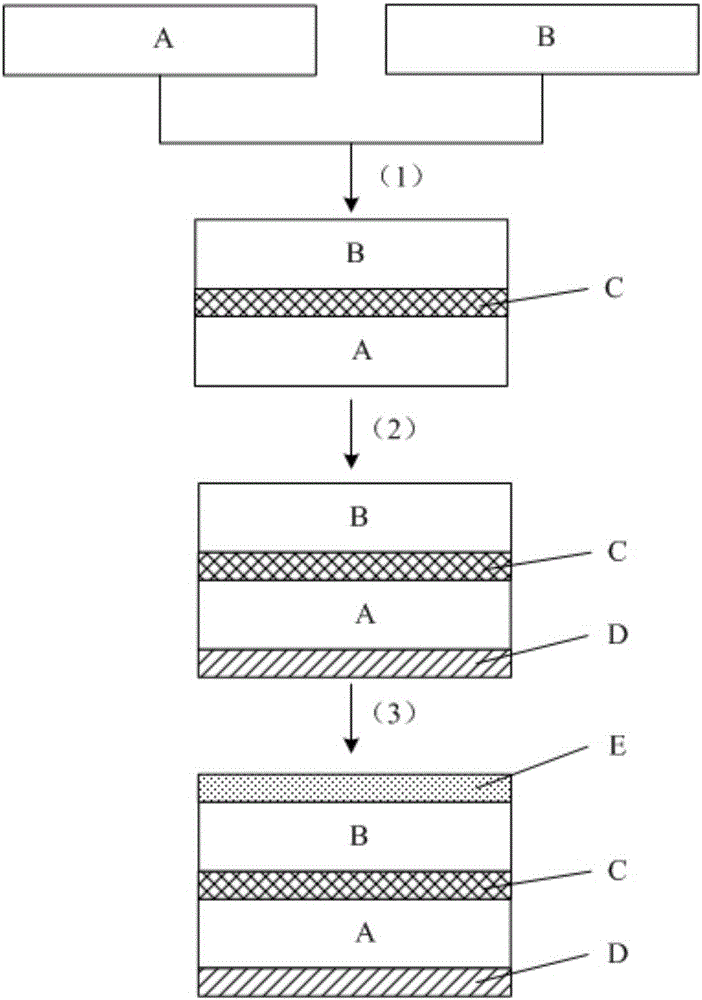
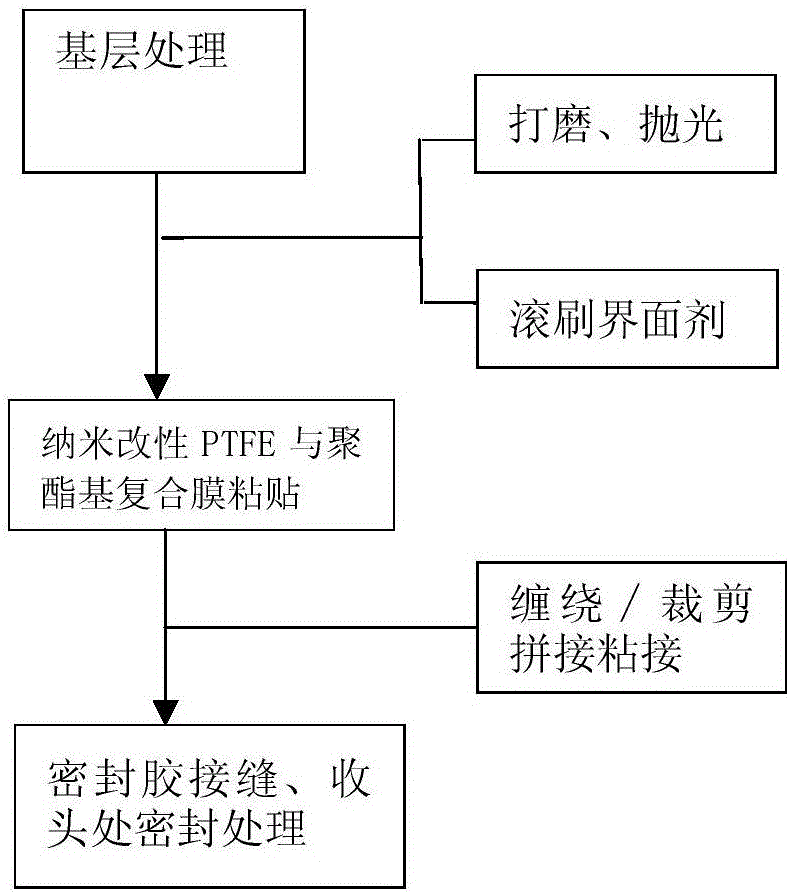
Preparation method and application of nano-modified PTFE and polyester-based composite film for preventing fan blades from icing
ActiveCN106313811ASolving non-adhesive technical problemsConvenient engineering constructionSynthetic resin layered productsLaminationPolyesterComposite film
The invention provides a preparation method and application of a nano-modified PTFE and polyester-based composite film for preventing fan blades from icing. The method includes the steps of PTFE film modification, lamination complexing and photo-crosslinked adhesive application. A modifier is prepared from antimony-doped tin oxide nano-crystals, nano-titanium dioxide, nano-silicon carbide, an organic fluorine waterproofing agent and pentaerythritol tri-(3-aziridinyl)-propionate; in lamination complexing, a bonding complexing agent is prepared from 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate, vinyl acetate, ethyl carbamate, alpha-linolenic acid, (2)ethoxylated bisphenol A dimethacrylate, trimethylolpropane triacrylate and benzoyl peroxide; a photo-crosslinked adhesive is prepared from a poly[butyl acrylate-glycidyl methacrylate-n-butoxy methacrylamide]copolymer, vinyl acetate, butyl acrylate, an acrylate derivative, a photoinitiator and dimethylformamide. The method and the composite film solve the non-adhesion problem that a PTFE film can not be pasted on the surfaces of fan blades with an adhesive directly.
Owner:NANJING HAOHUI HI TECH CO LTD
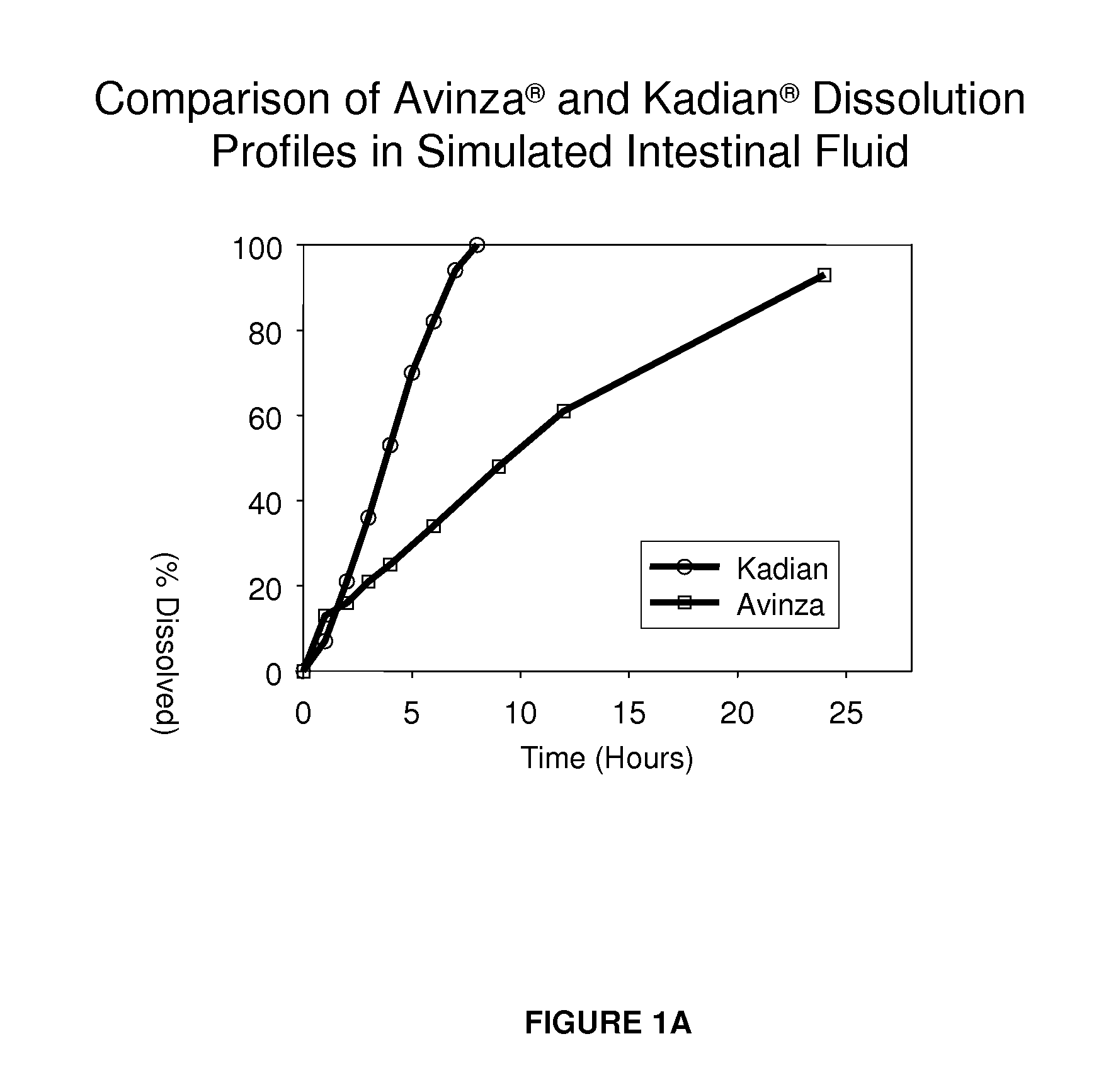
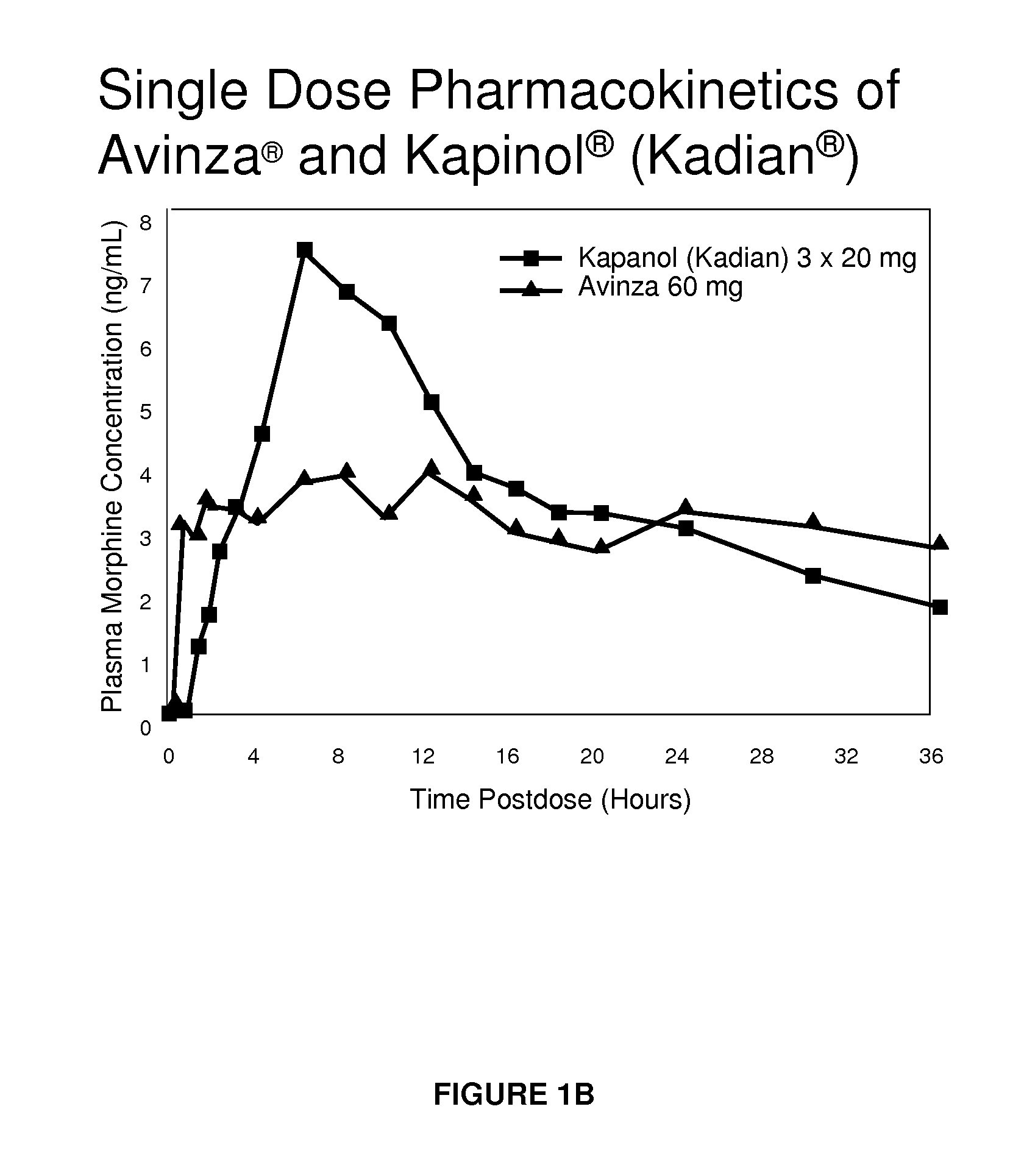
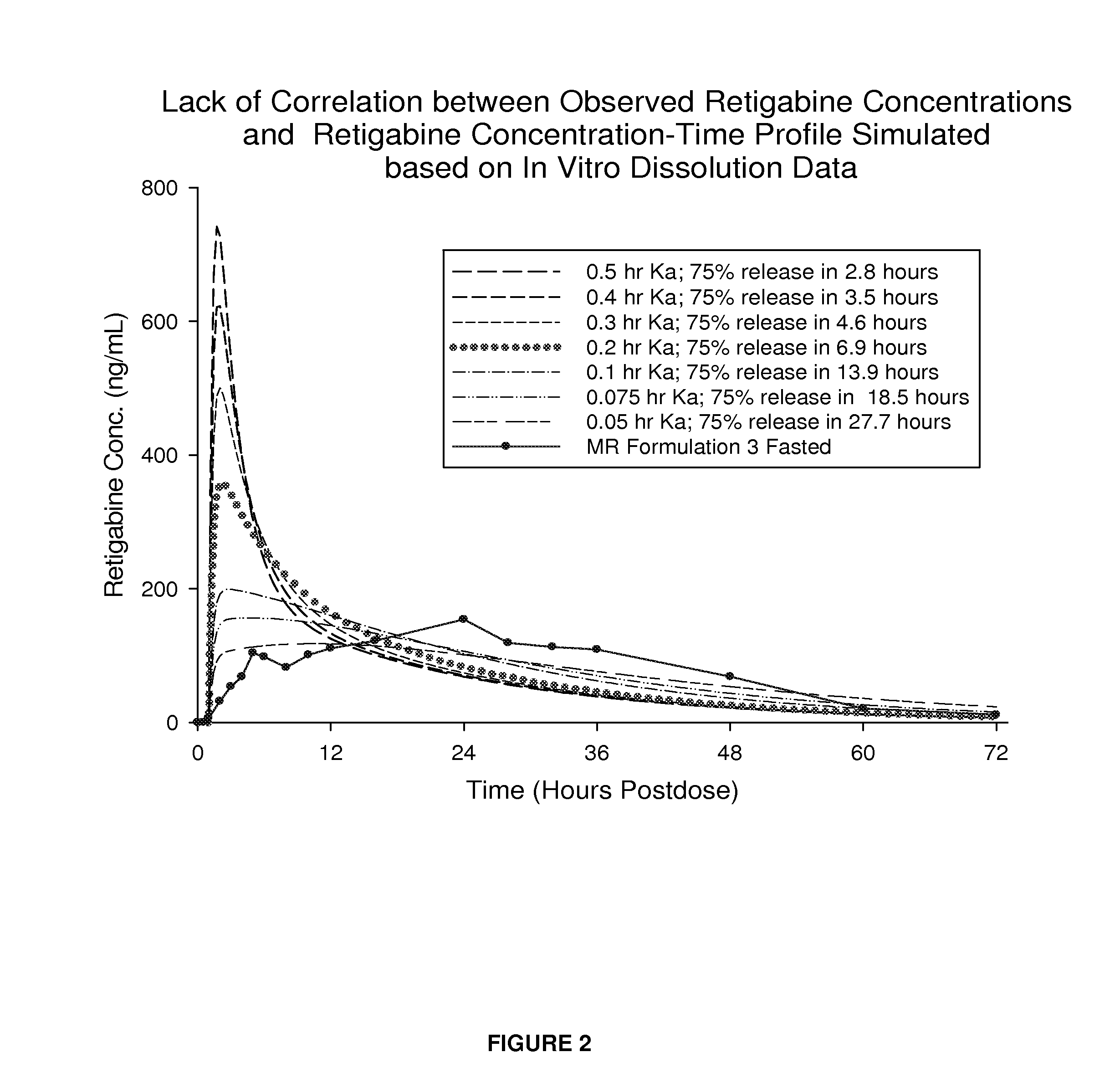
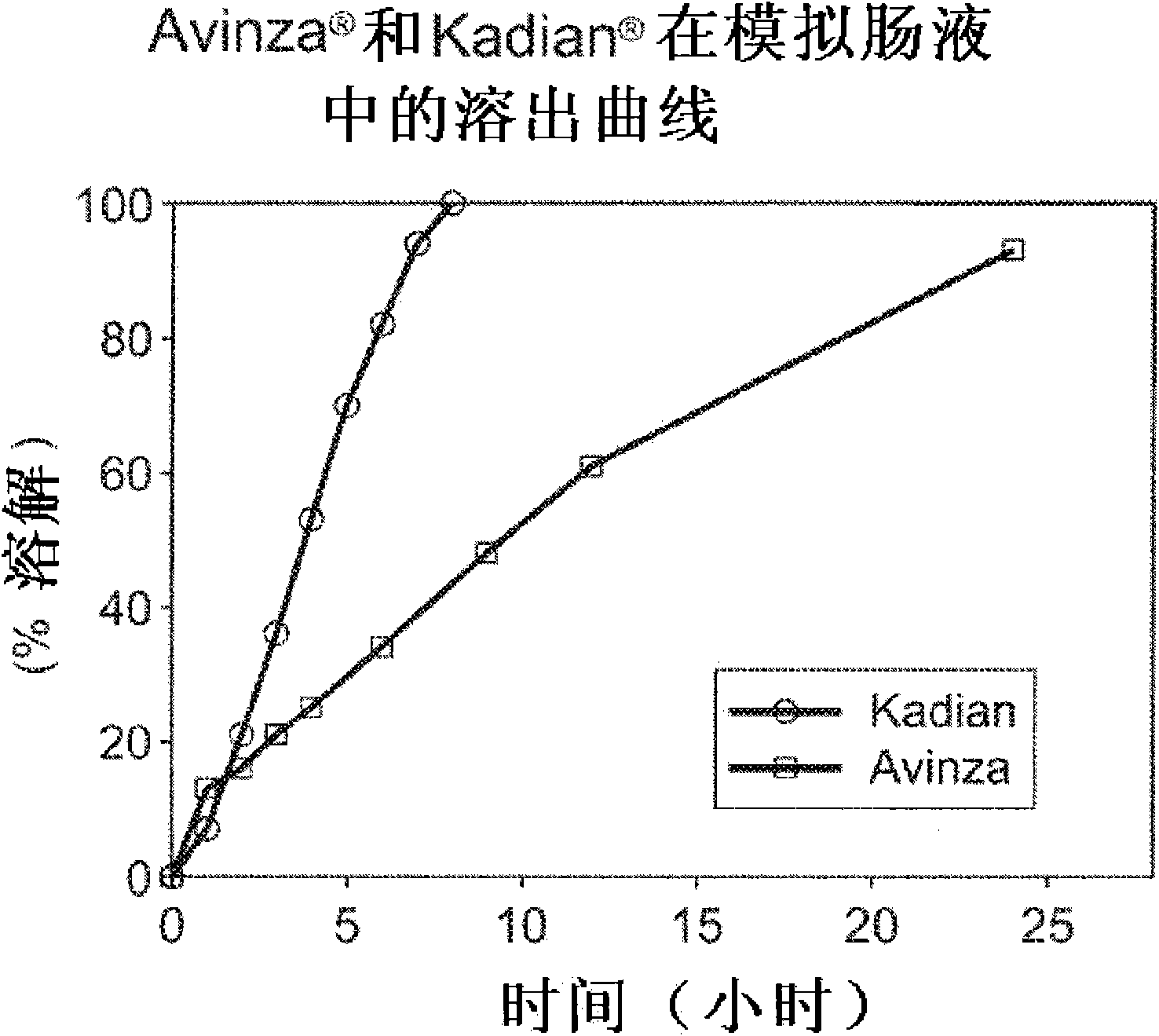
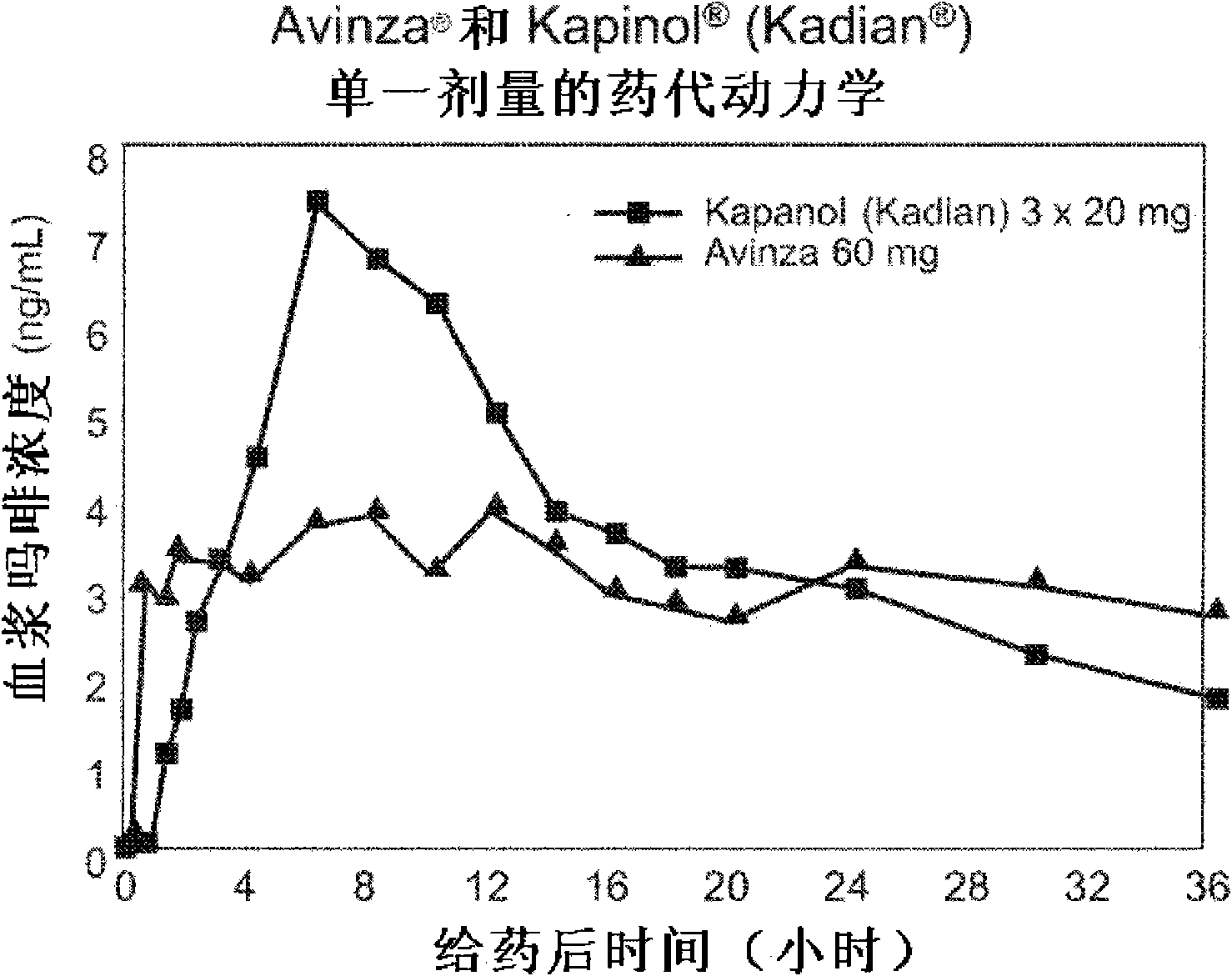
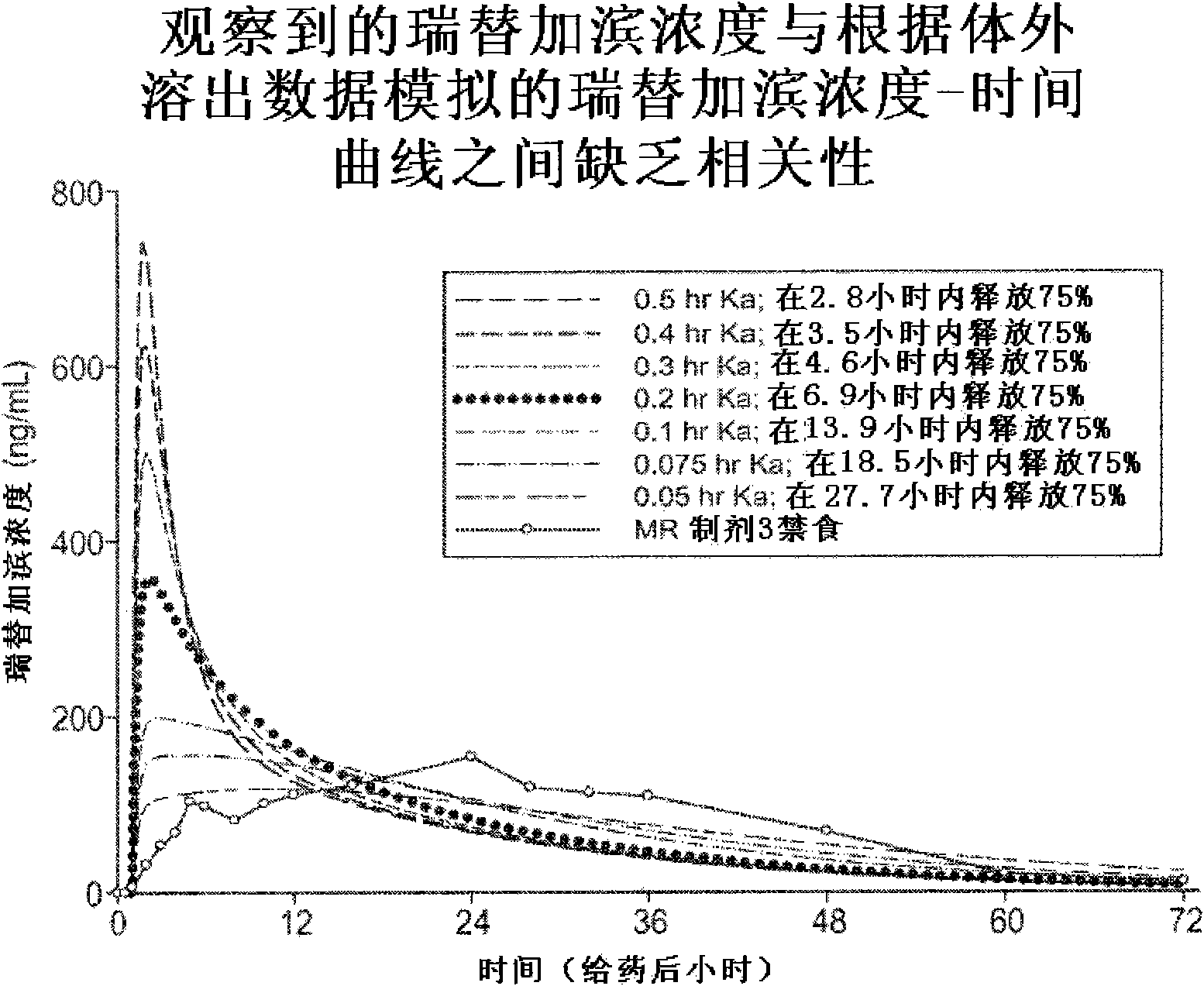
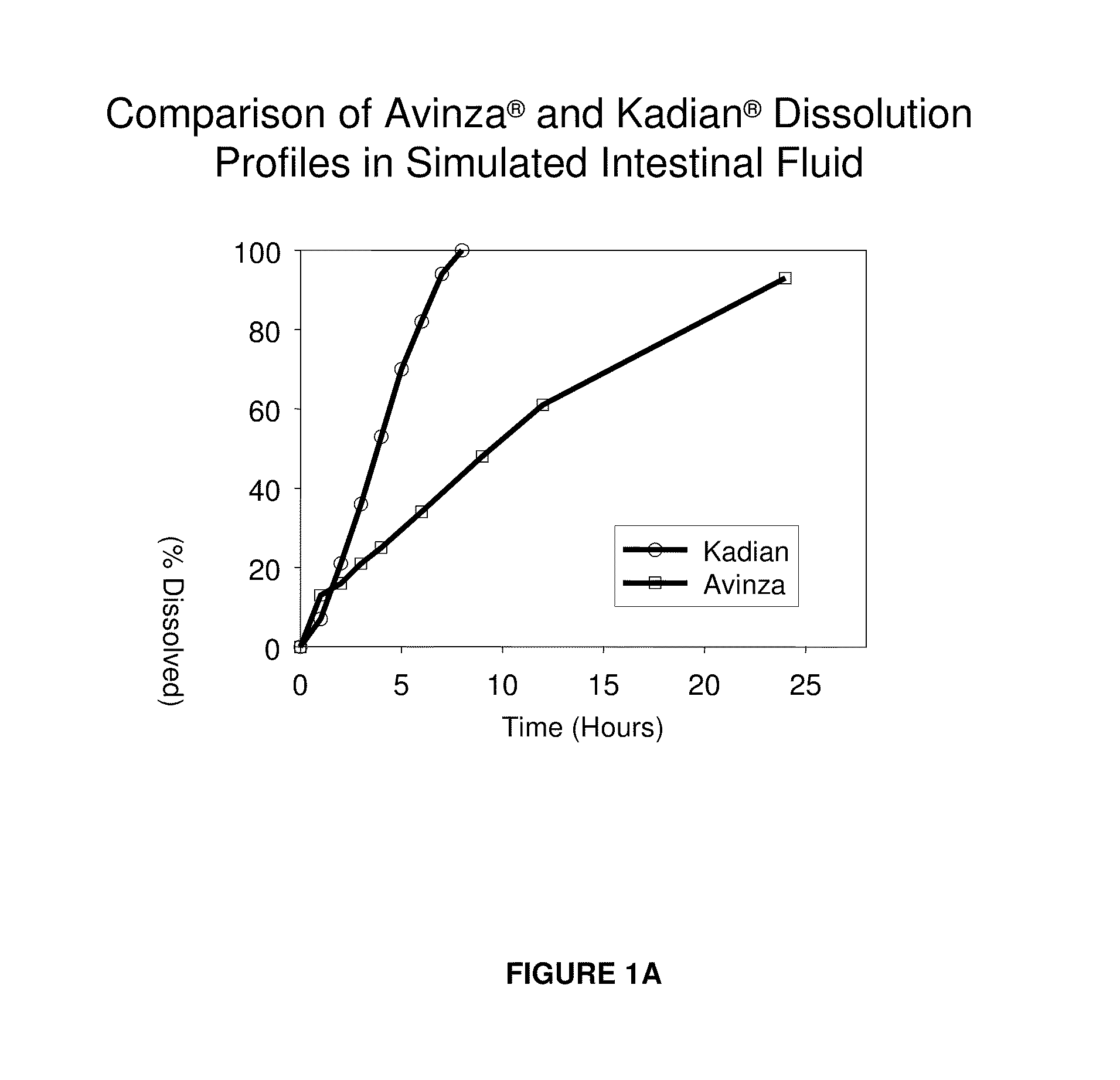
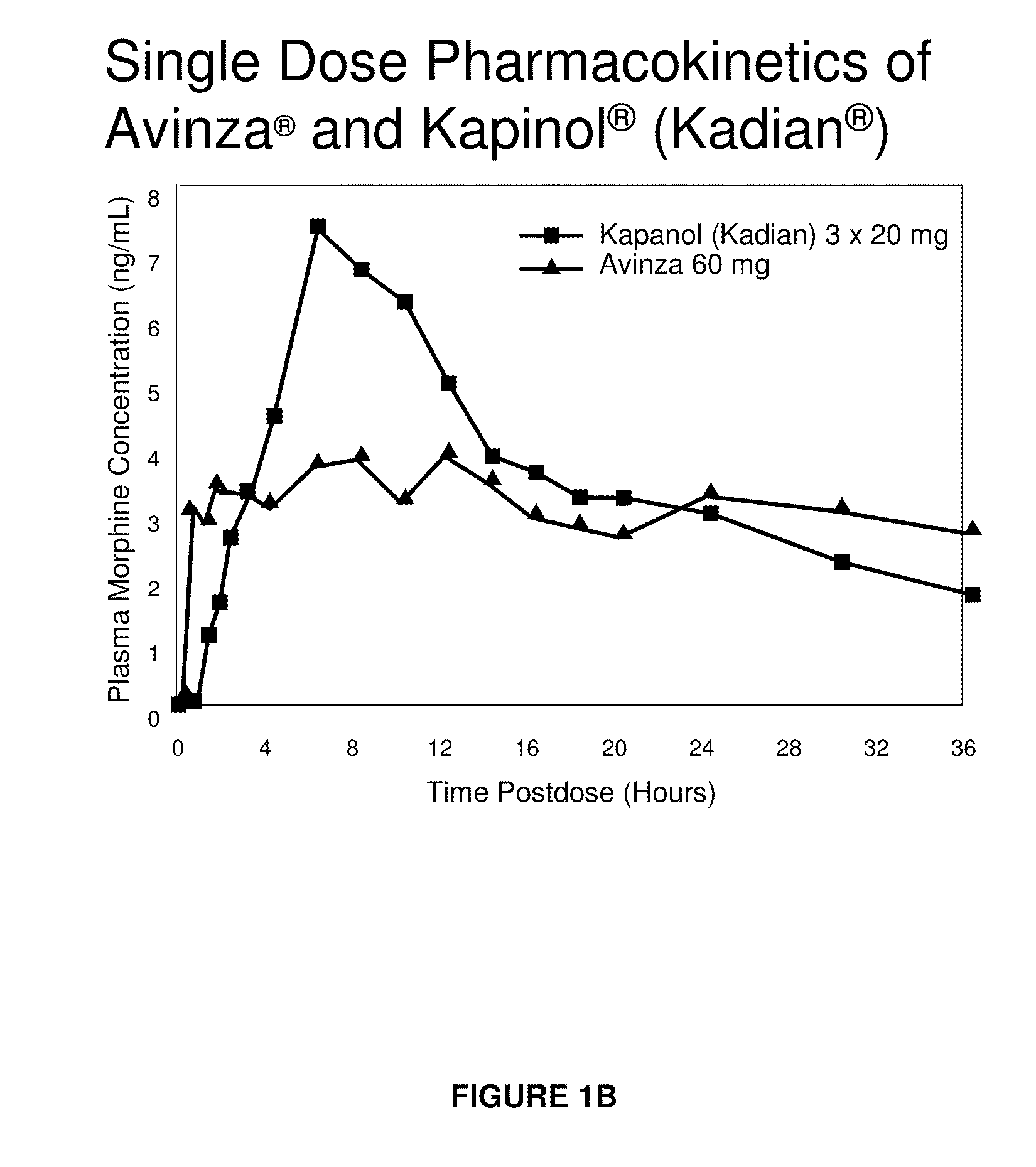
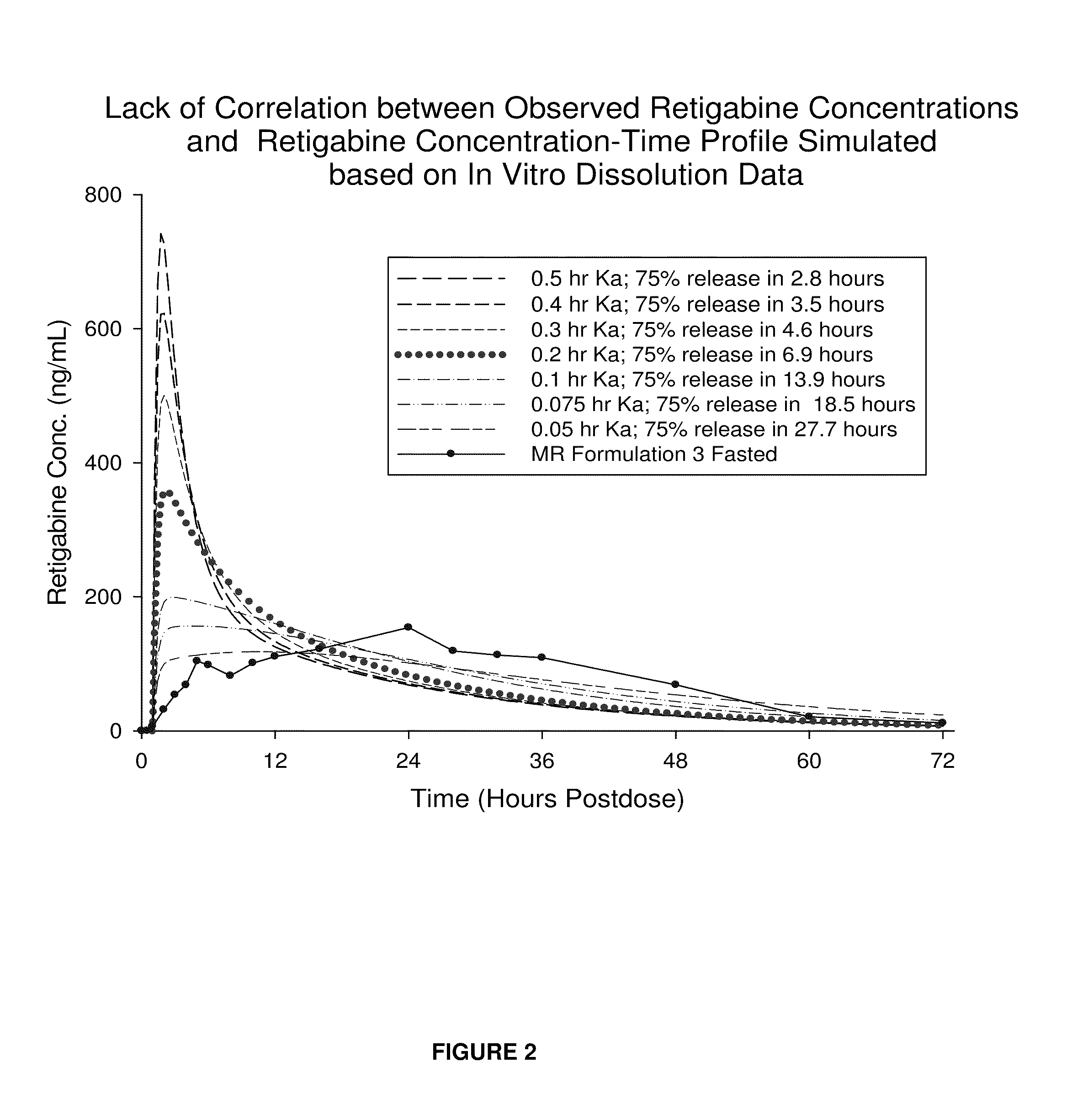
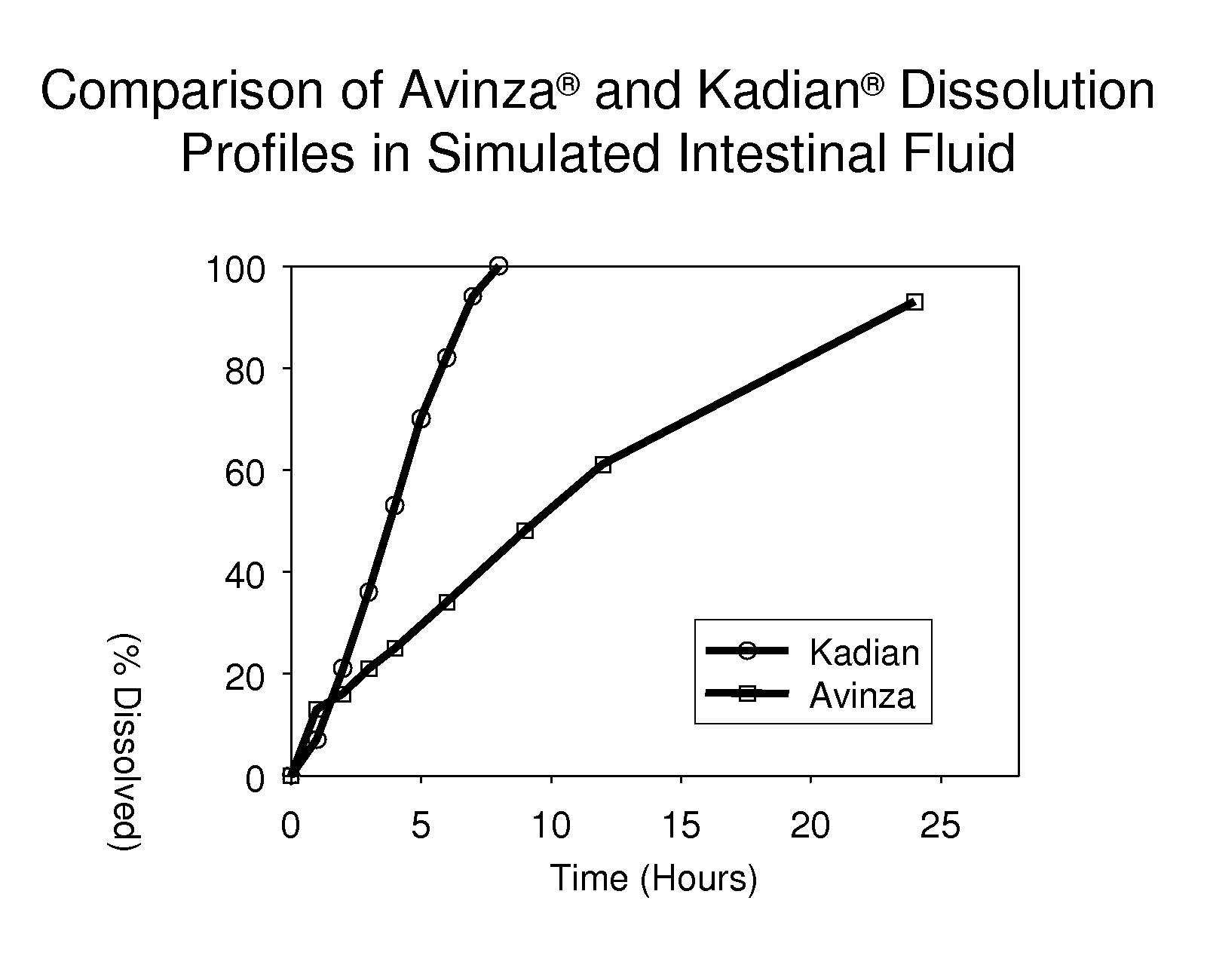
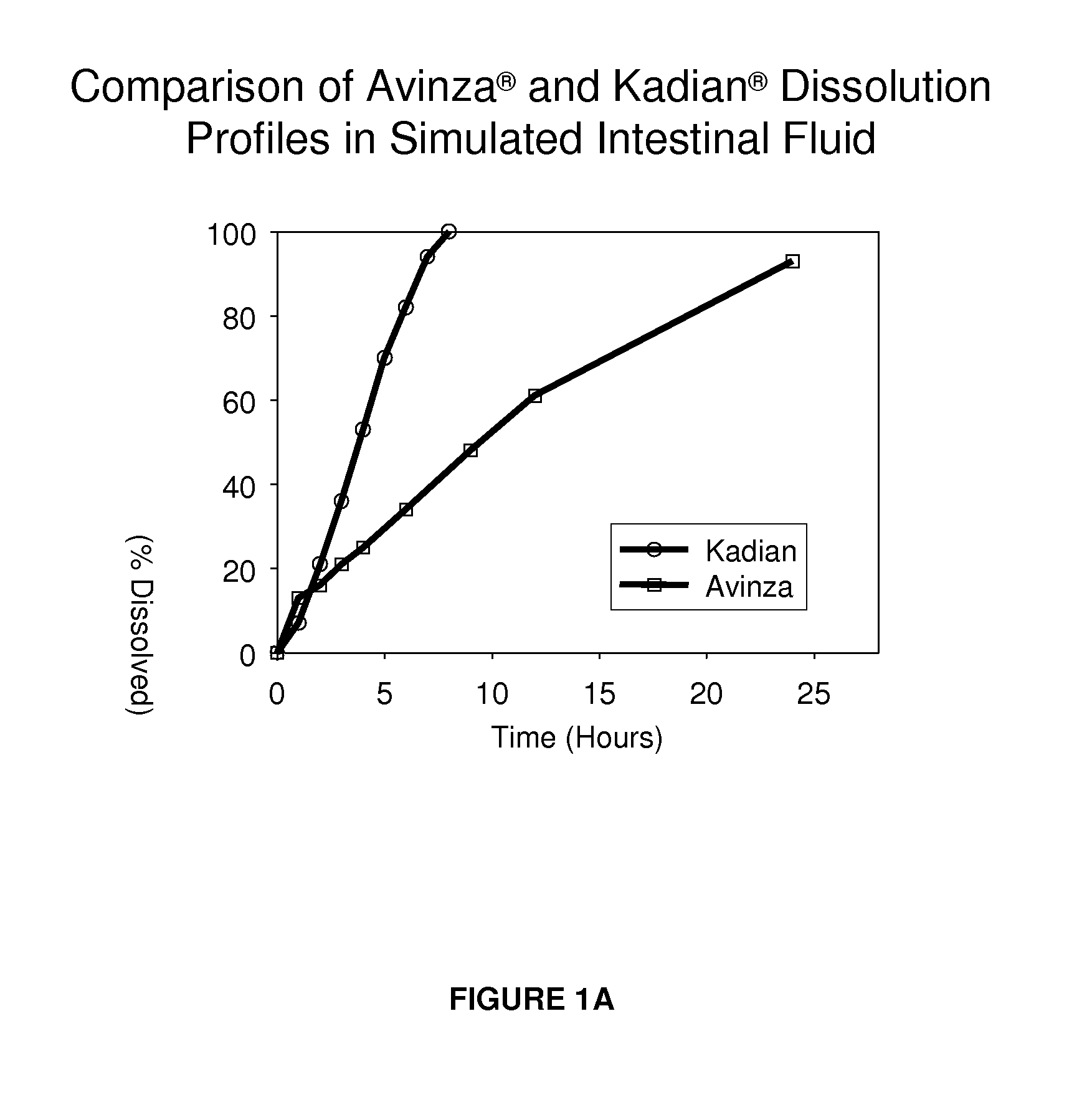
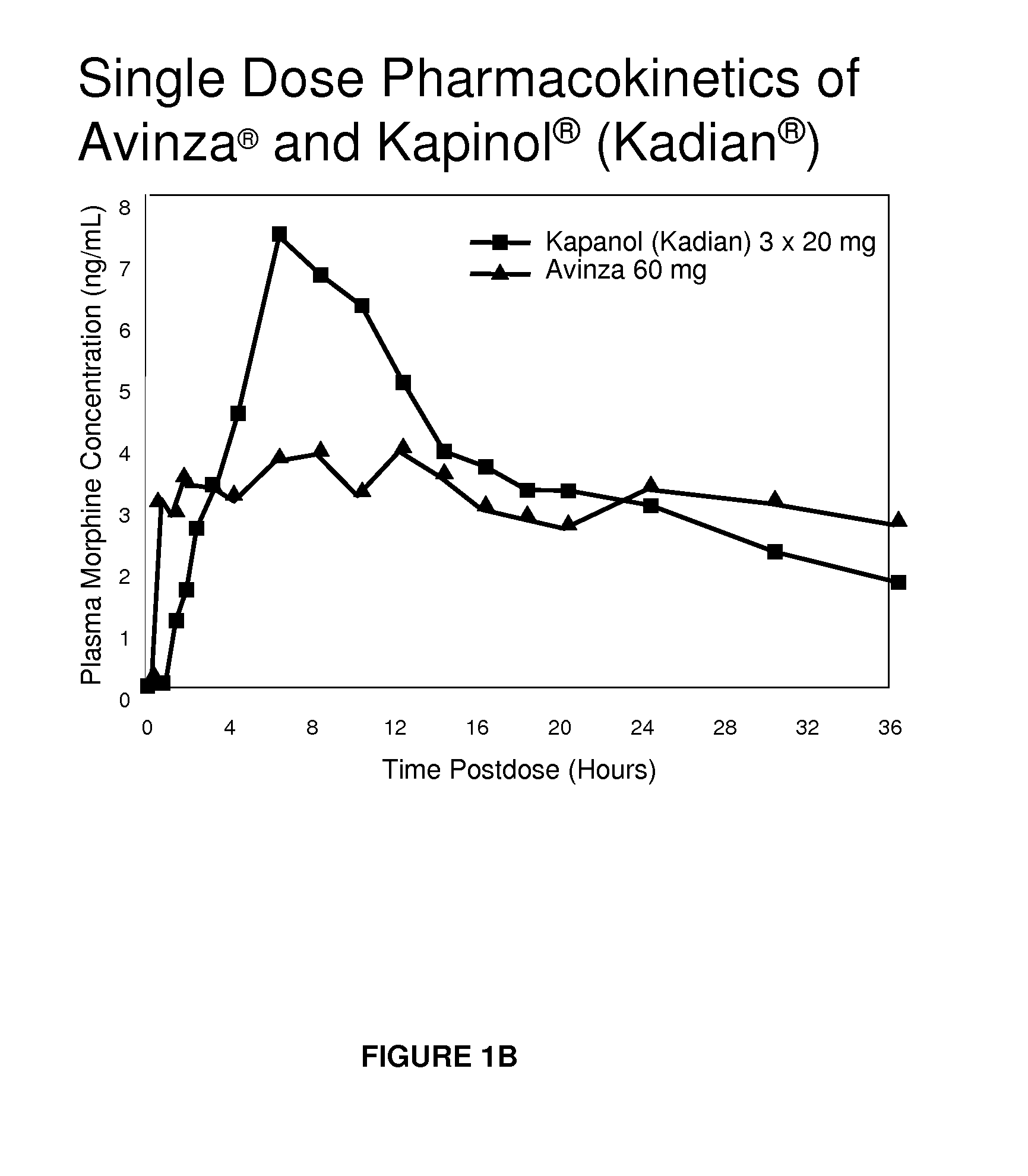
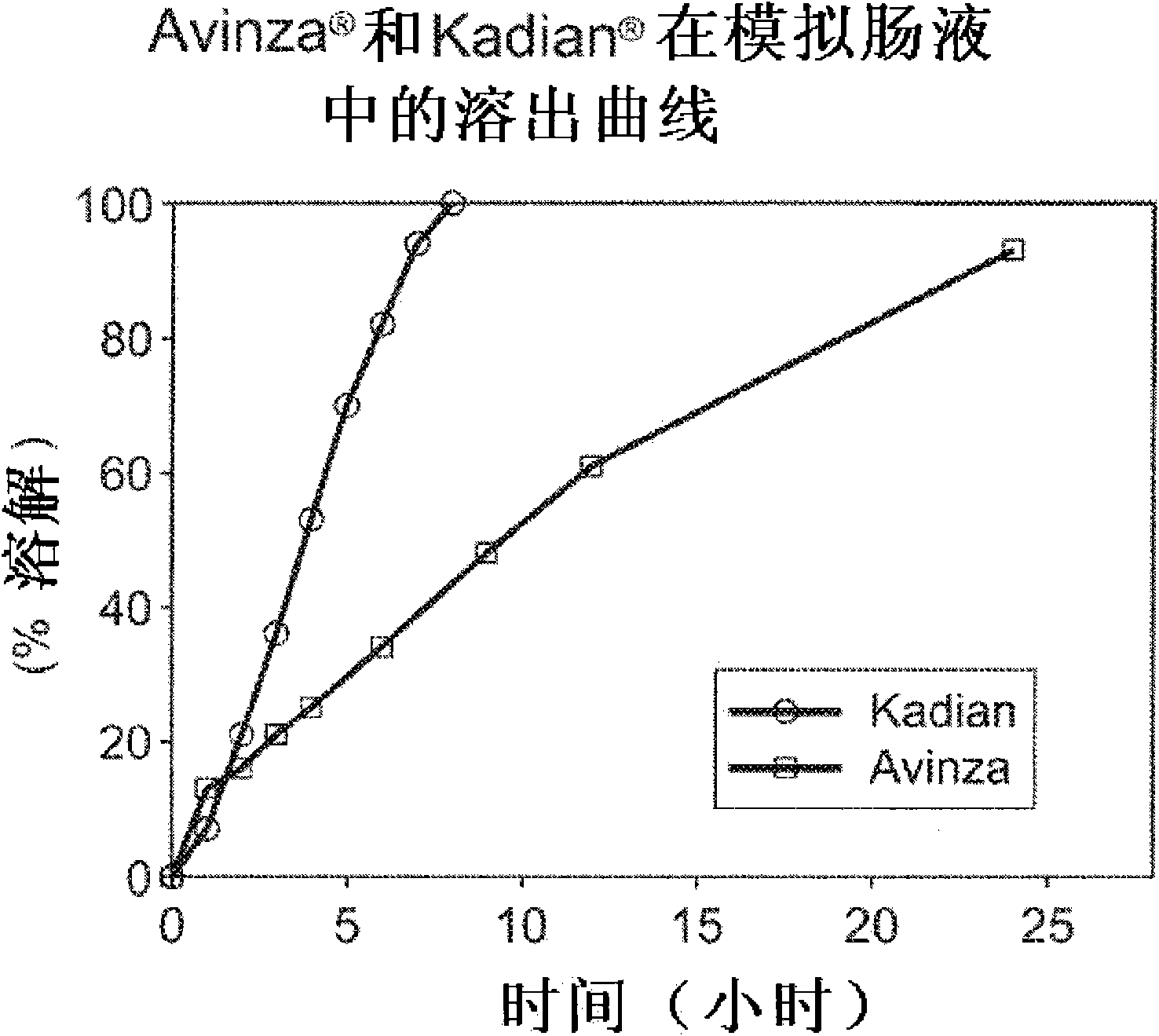
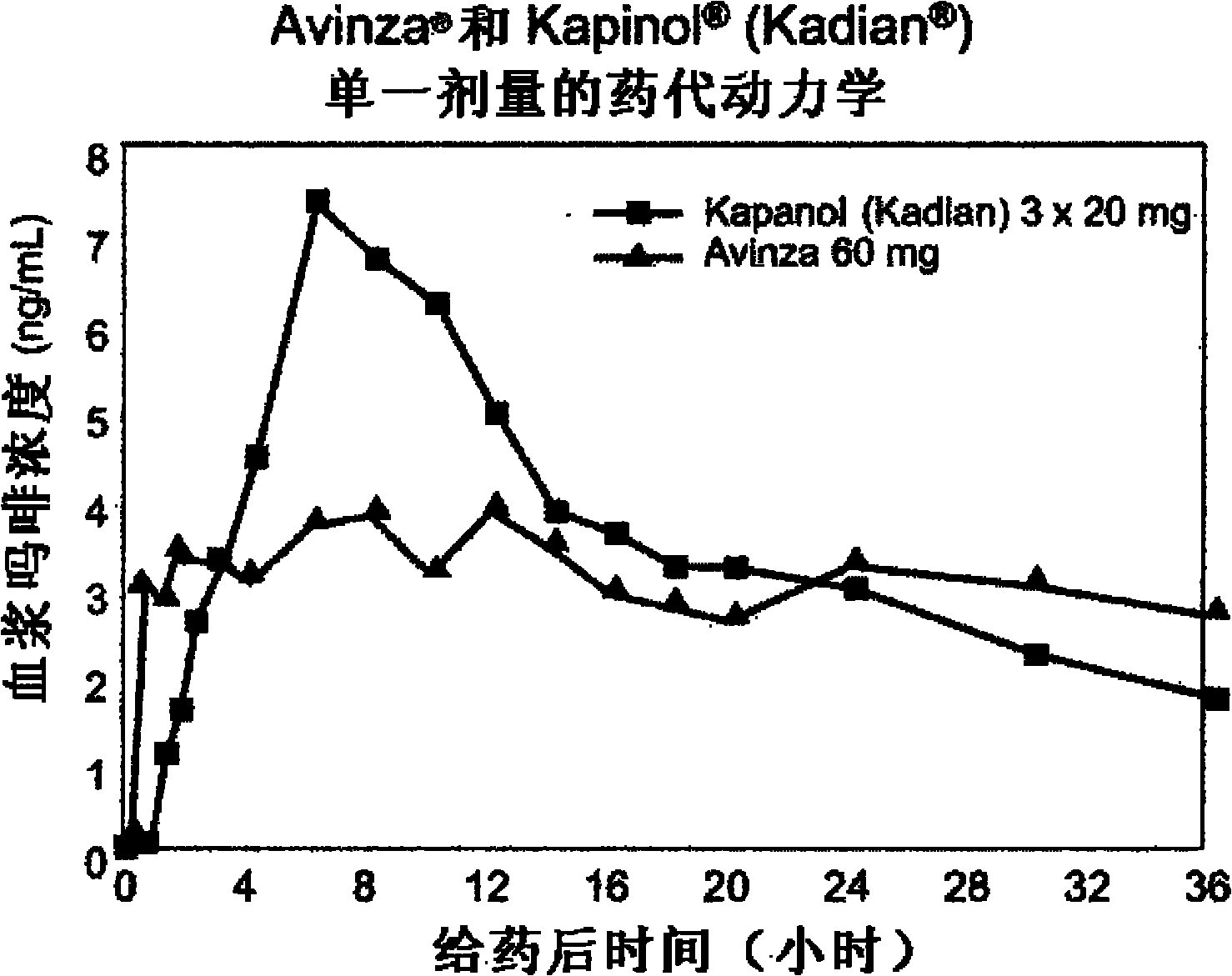
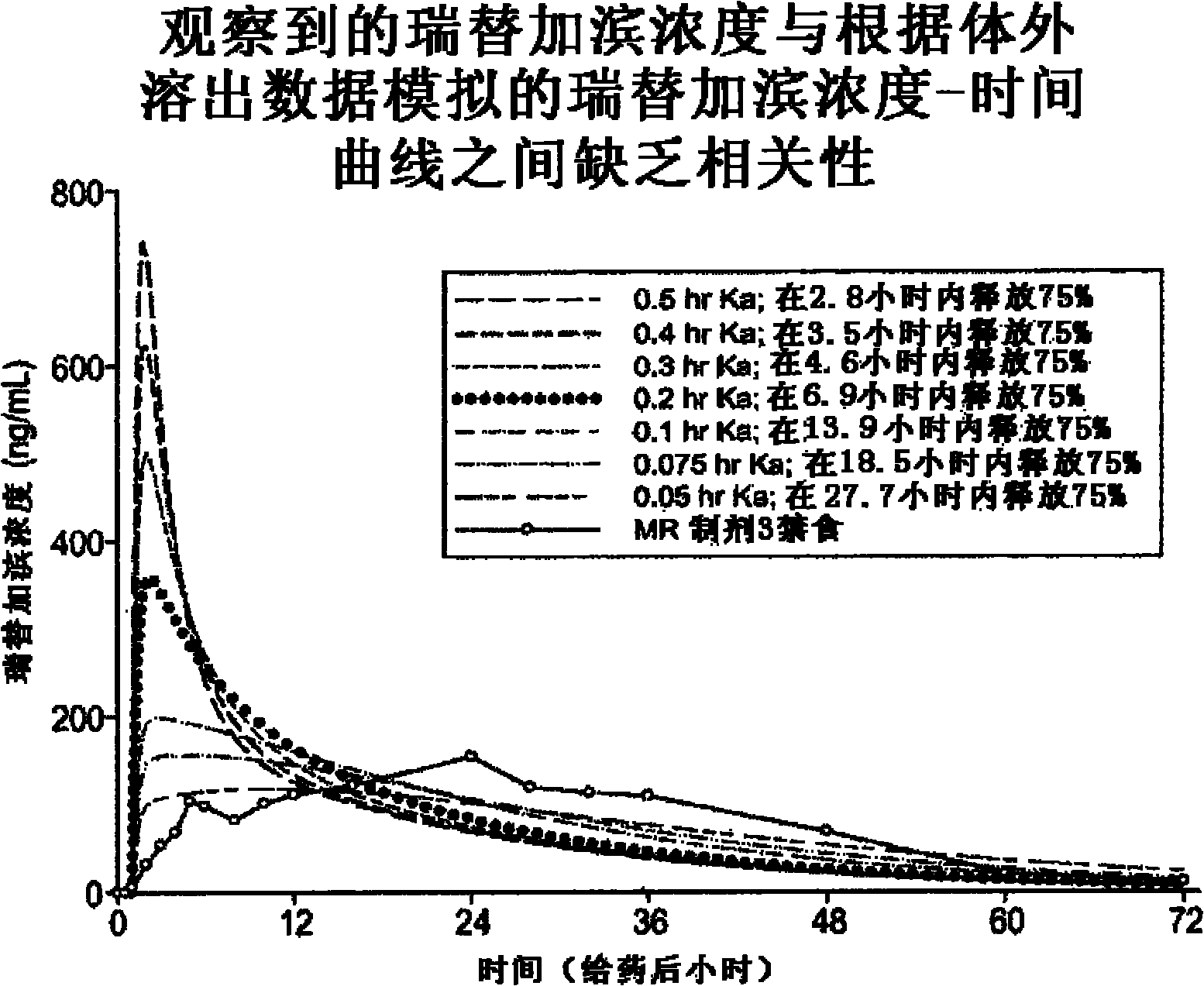
Modified release formulation and methods of use
A modified release pharmaceutical formulation includes about 30-70% N-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix including hydroxypropylmethylcellulose (HPMC), and an enteric polymer. The pharmaceutical formulation produces a sustained plasma concentration of retigabine following administration to a subject for 4-20 hours longer than the time required for in vitro release of 80% of retigabine. The plasma concentration vs. time profile of this formulation is substantially flat over an extended period lasting for about 4 hours to about 36 hours. A method of treating a disorder characterized by nervous system hyperexcitability includes administering to a subject an effective amount of these pharmaceutical formulations.
Owner:VALEANT PHARMA INT
Modified release formulation and methods of use
A modified release pharmaceutical formulation includes about 30-70% N-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix including hydroxypropylmethylcellulose (HPMC), about 1.0-10% of an anionic surfactant, and an enteric polymer. The pharmaceutical formulation produces a sustained plasma concentration of retigabine following administration to a subject for 4-20 hours longer than the time required for in vitro release of 80% of retigabine. A formulation includes about 30-70% N-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix, and an agent for retarding release in the gastric environment. The plasma concentration vs. time profile of this formulation is substantially flat over an extended period lasting for about 4 hours to about 36 hours. A method of treating a disorder characterized by nervous system hyperexcitability includes administering to a subject an effective amount of these pharmaceutical formulations.
Owner:VALEANT PHARMA INT
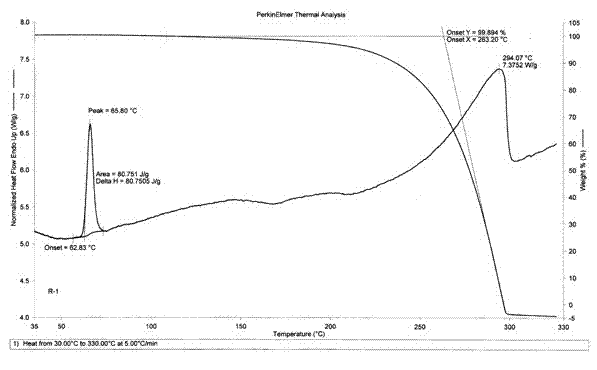
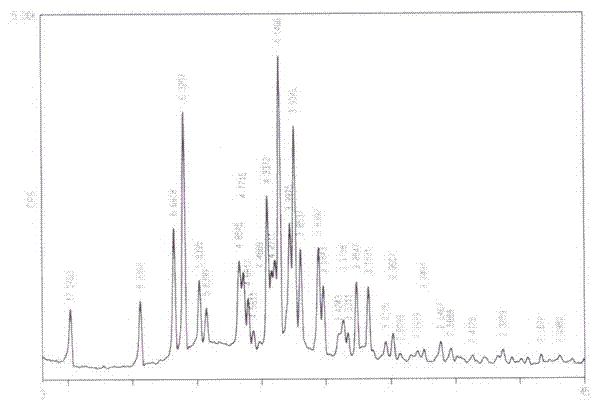
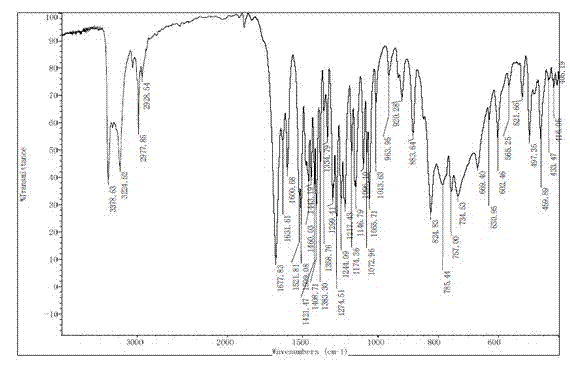
Novel crystal form D of Retigabine and preparation method thereof
ActiveCN102531966AImprove securityLarge particle sizeNervous disorderCarbamic acid derivatives preparationPhysical chemistryPharmaceutical drug
The invention relates to the field of medicinal chemistry, in particular to a novel crystal form D of N-(2- amino-4-(4- fluoro benzyl amino) phenyl) ethyl carbamate (Retigabine) and a preparation method of the novel crystal form D. The invention provides X-ray powder diffraction characteristic absorption peaks and DSC (differential scanning calorimetry) endothermic transition peaks of the novel crystal form. The novel crystal form D of the Retigabine is characterized in that the crystal has characteristic absorption peaks at angles of (2 theta) 5.18 DEG, 10.60 DEG, 13.22 DEG, 13.98 DEG, 15.70DEG, 18.26 DEG, 20.46 DEG, 21.40 DEG, 22.22 DEG, 22.58 DEG, 23.06 DEG, 24.44 DEG, 24.82 DEG, 27.38 DEG and 28.28 DEG under the X-ray powder diffraction and the DSC endothermic transition of the novelcrystal form is at a temperature of 60-70 DEG C. The novel crystal form D of the Retigabine is thin in grain size (the average grain size is 20-35 mum and normal distributed), and can be used for medicinal preparation production with no need for physical smashing, therefore, the production link is decreased, the production cost is reduced, the pollution and the medicinal property changing caused in smash process are avoided and the clinical medication safety is increased.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
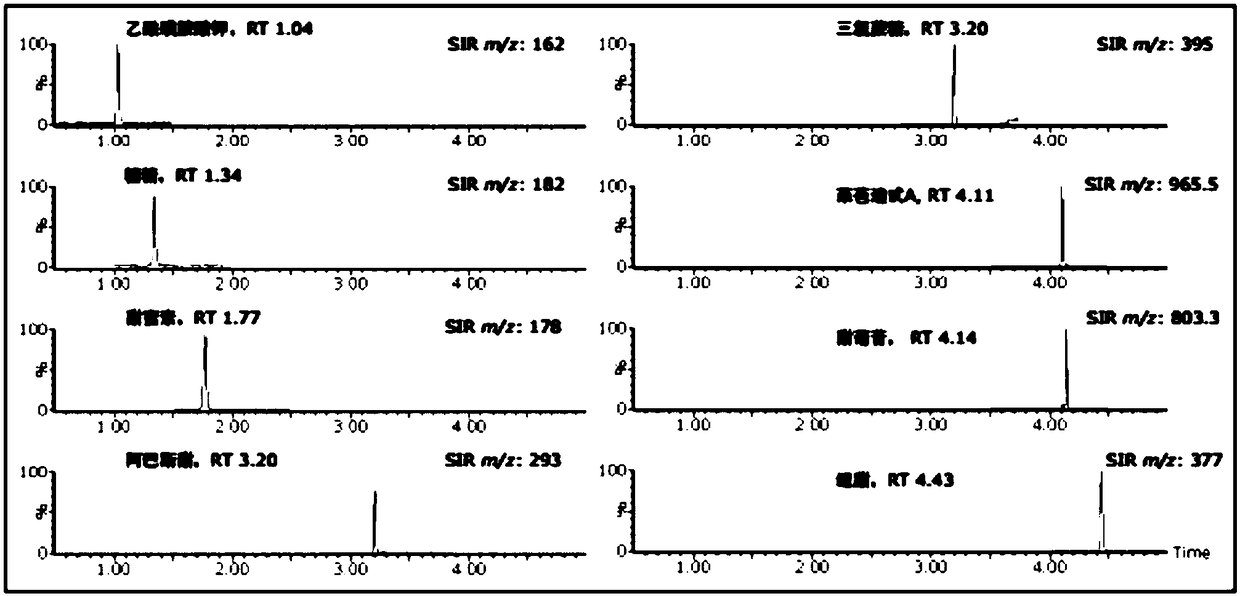
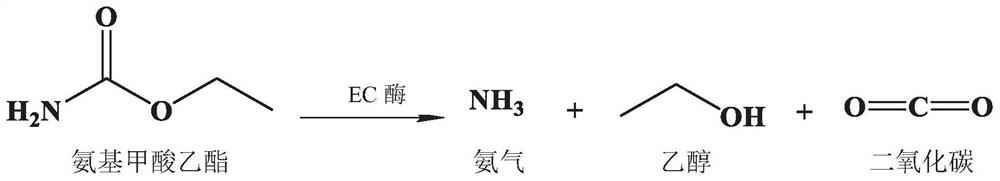
Method for rapidly simultaneously detecting eight sweeteners and urethane in baijiu by UPLC serially connecting QDa
InactiveCN108627586AMeet the low limit detection requirementsQuick checkComponent separationCyclamatesData acquisition
The invention discloses a method for rapidly simultaneously detecting eight sweeteners and urethane in baijiu by UPLC serially connecting QDa. The method comprises the steps of pre-treating a baijiu sample to be tested, separating by ultra-high performance liquid chromatography, and carrying out data acquisition and detection with dual channels of a QDa detector. The method is simple, rapid, accurate and reliable, and can be used for simultaneously detecting the content of 8 sweeteners (acesulfame, sodium saccharin, cyclamate, aspartame, neotame, rebaudioside A, stevioside, and sucralose) andurethane in baijiu.
Owner:ANHUI GUJING DISTILLERY
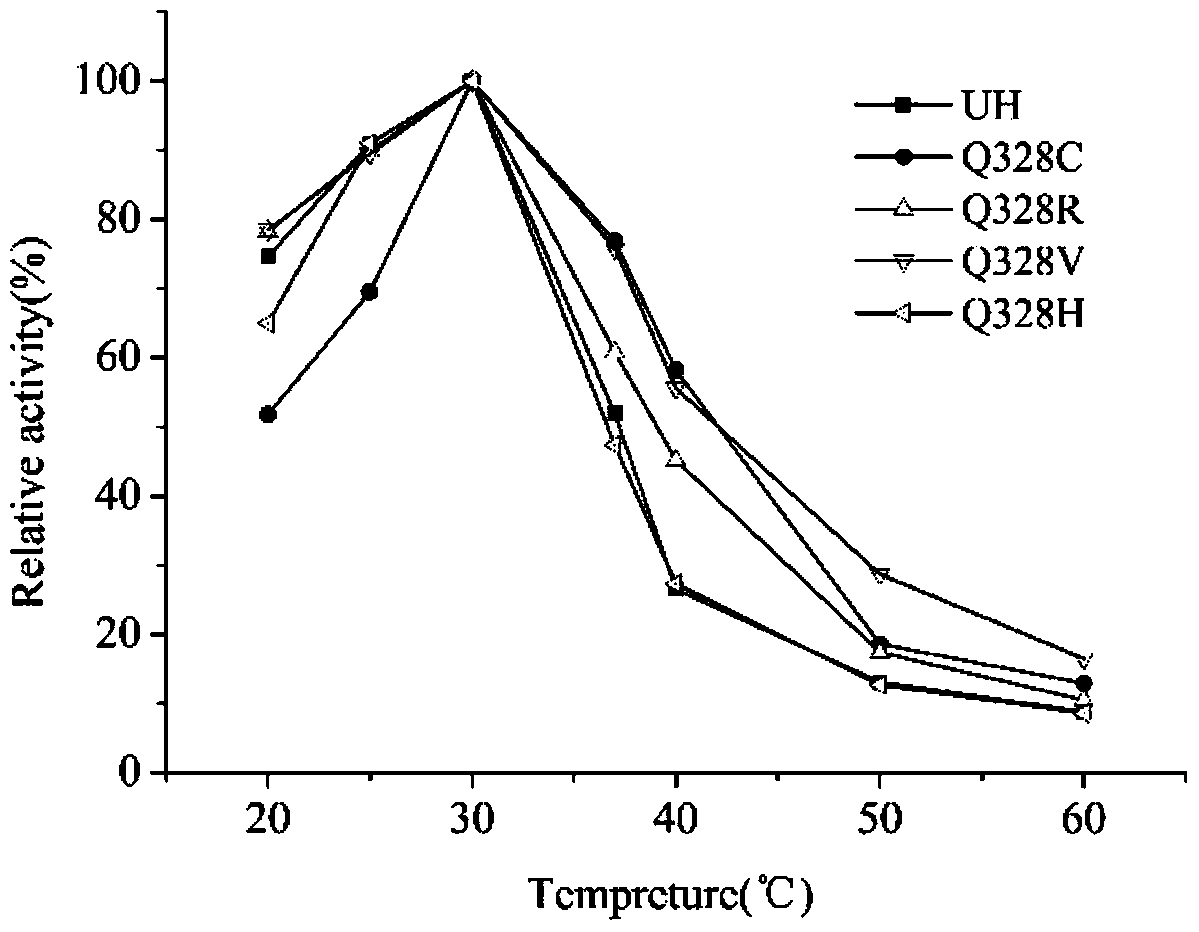
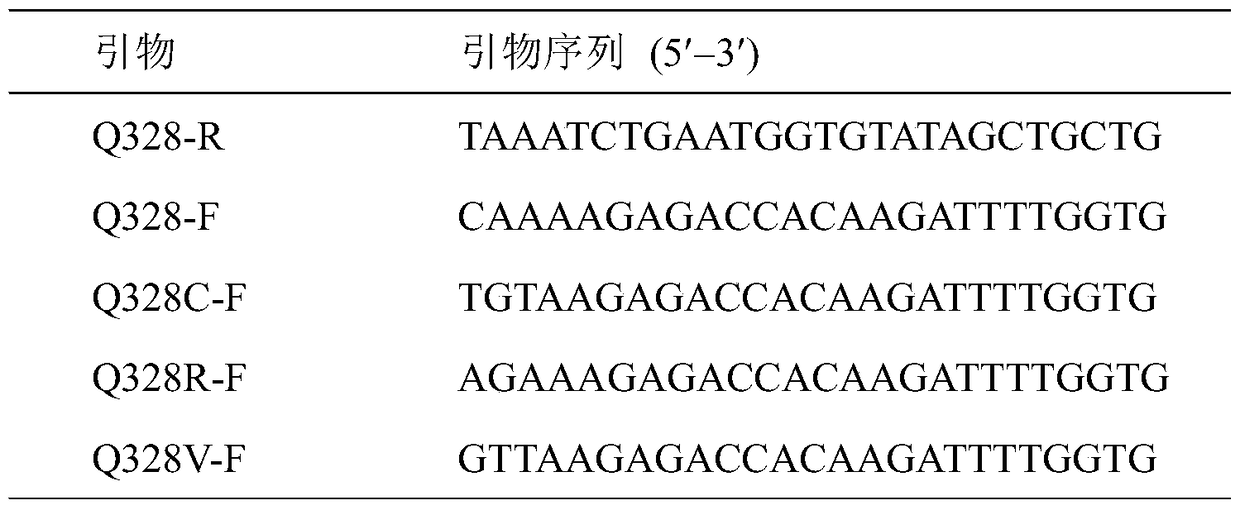
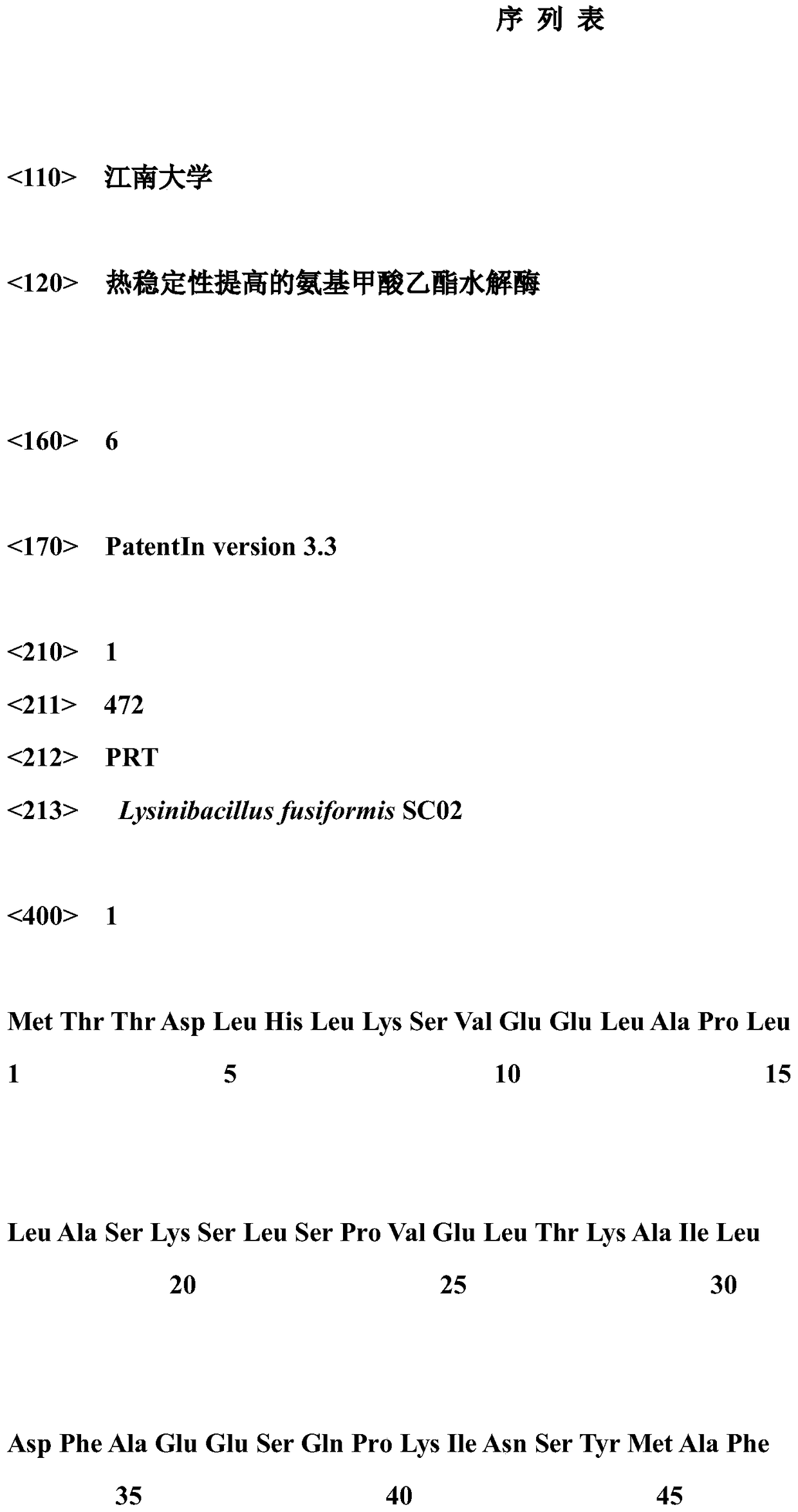
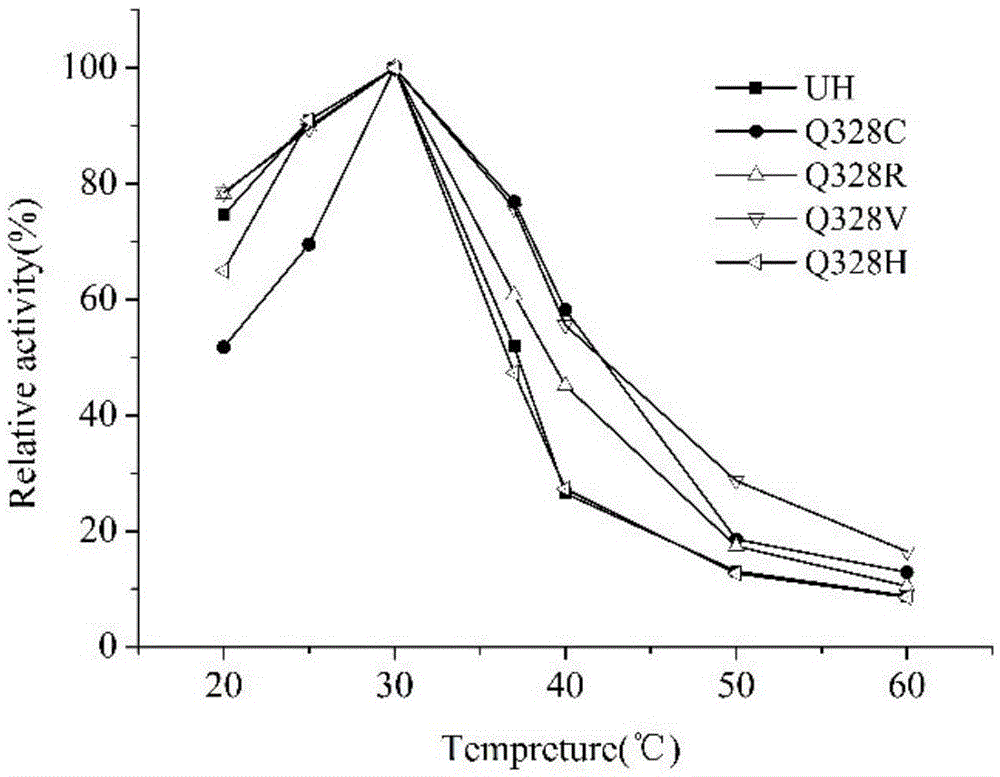
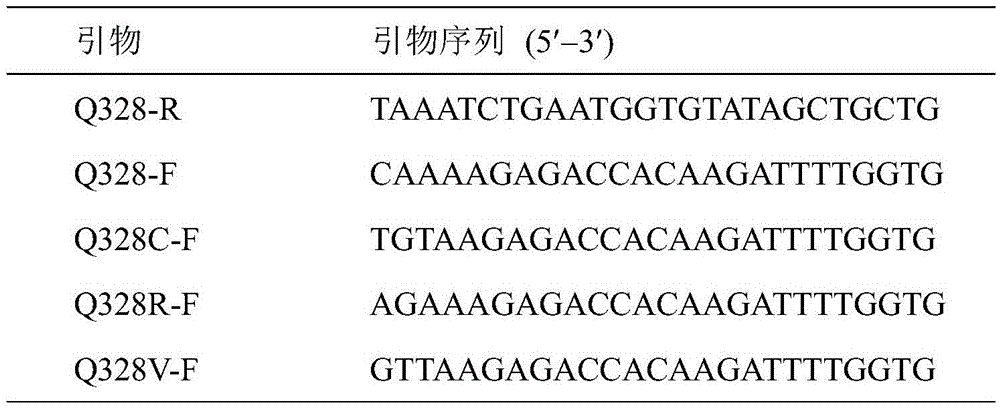
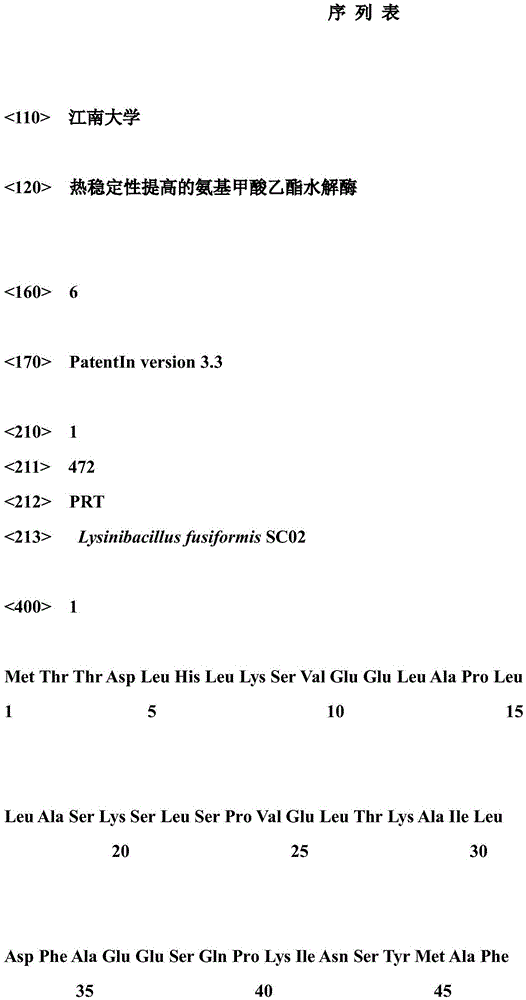
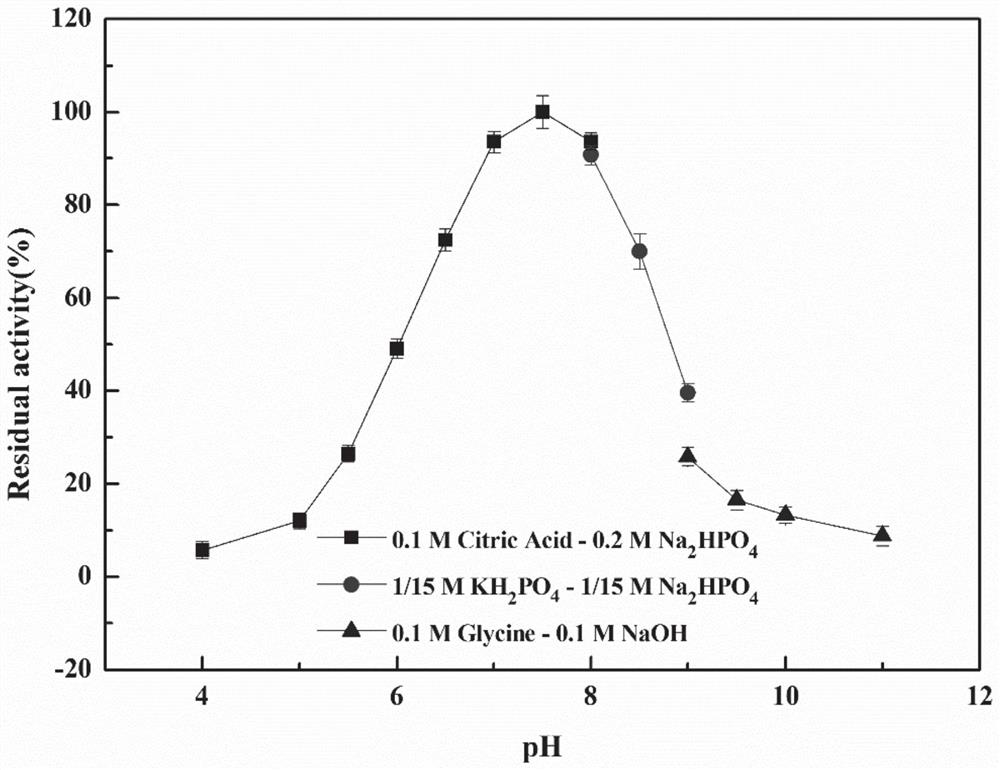
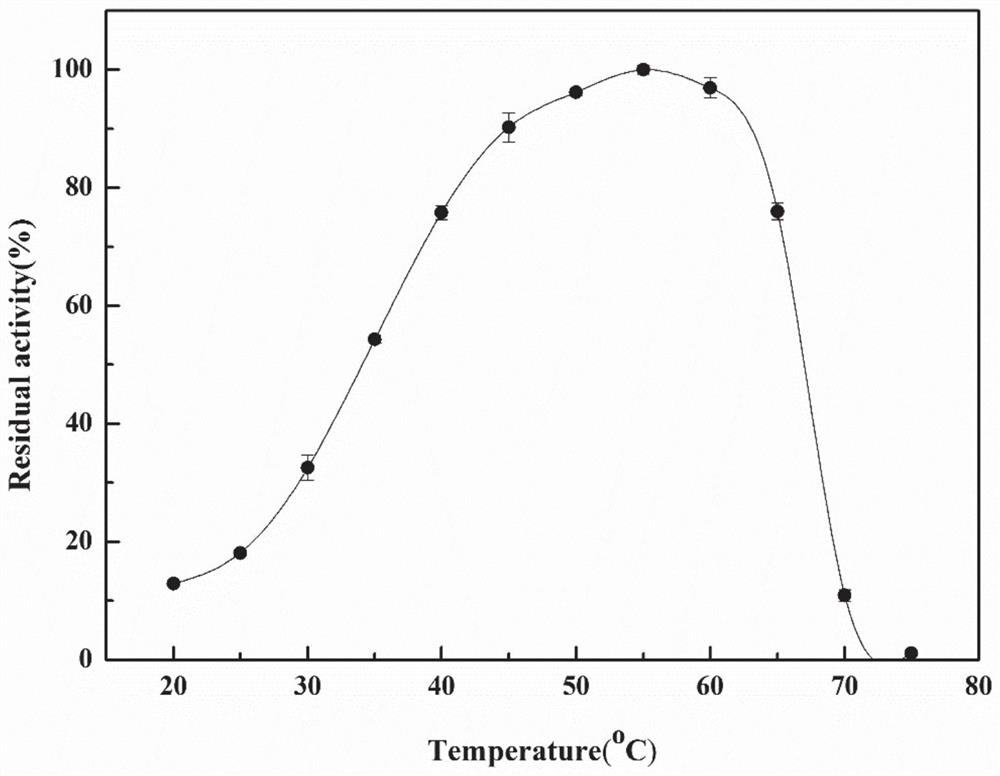
Ethyl carbamate hydrolytic enzyme mutants capable of improving thermostability
ActiveCN105420210APromote degradationImprove thermal stabilityHydrolasesAlcoholic beverage preparationAlcoholHalf-life
The invention discloses ethyl carbamate hydrolytic enzyme mutants capable of improving the thermostability, and belongs to the technical field of gene engineering and enzyme engineering. By means of a molecular technical means, the ethyl carbamate hydrolytic enzyme mutants capable of improving the thermostability are obtained, and the half-life periods of the ethyl carbamate hydrolytic enzyme mutants Q328C and Q328V are increased by 7.46 times and 1.96 times respectively compared with a native enzyme. The mutants Q328C, Q328R and Q328 V all have better tolerance at 30 DEG C or above than the native enzyme. In addition, the tolerance to ethyl alcohol and the tolerance to acid of the mutant Q328C are improved.
Owner:JIANGNAN UNIV
Ethyl carbamate hydrolase mutant and application thereof
ActiveCN112342209AIncrease enzyme activityImprove toleranceHydrolasesBiofuelsAmidase activityEthyl ester
The invention discloses an ethyl carbamate hydrolase mutant and application thereof. The ethyl carbamate hydrolase mutant is obtained by mutation of an amidase gene of Agrobacterium tumefaciens d3, the amino acid sequence of wild amidase is shown as SEQ ID No.1, and the ethyl carbamate hydrolase mutant is obtained by mutation of at least one site of R94P, I97L, S177C or G195A of the wild amidase.The amidase from the Agrobacterium tumefaciens d3 is obtained by screening, the residual enzyme activity is higher than 90% when the ethanol concentration is between 0% and 20%, the enzyme activity isquickly reduced when the ethanol concentration is higher than 25% , and the amidase shows excellent tolerance to low-concentration ethanol. Through semi-rational transformation, a mutant library with21 point mutations is constructed, four mutation sites (R94P, I97L, S177C and G195A) with improved enzyme activity are screened from the mutant library, and the enzyme activity of a combined mutant strain I97L / G195A is improved by 5.2 times and is improved to 2442U / L from original 395U / L.
Owner:ZHEJIANG UNIV
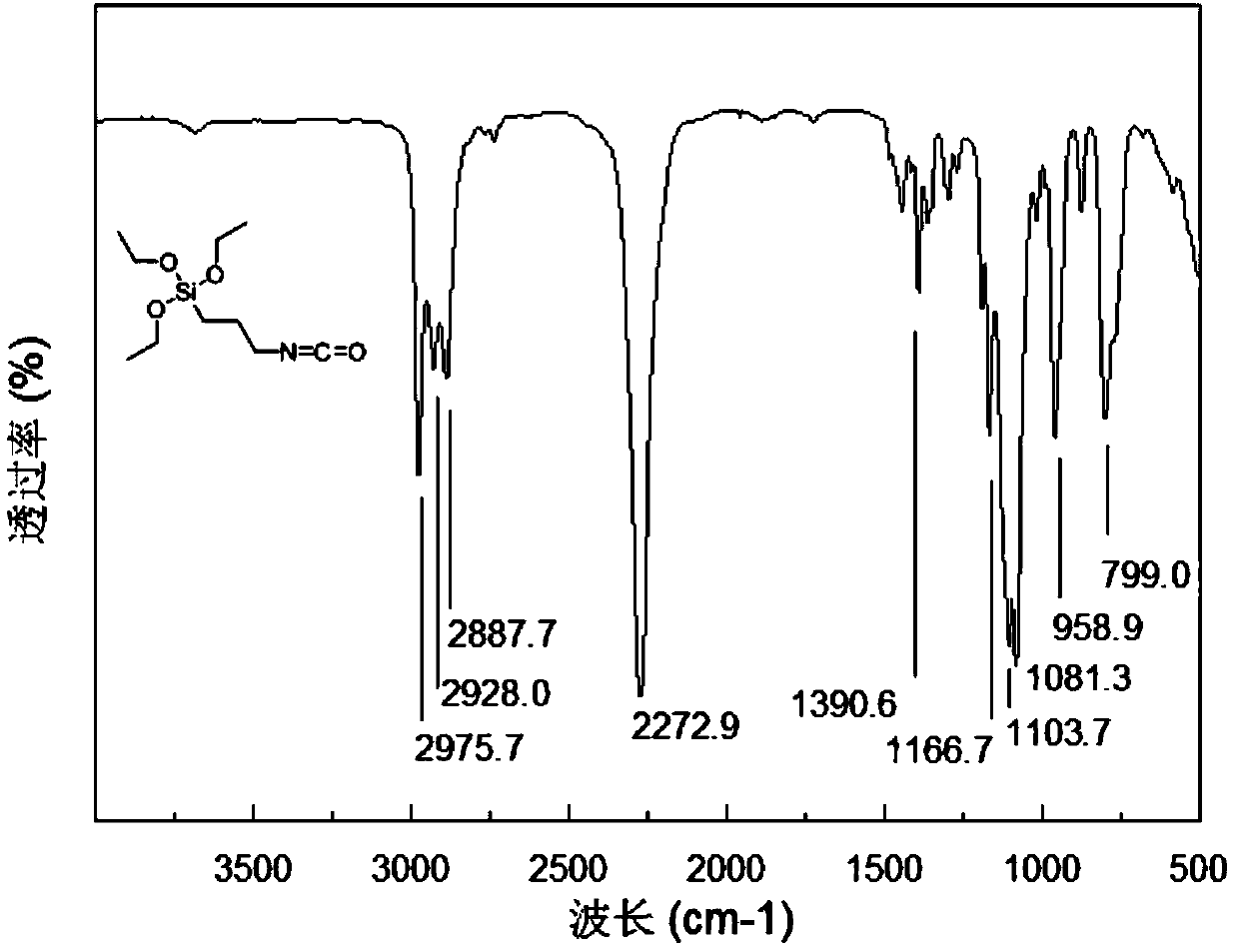
Synthesizer for high-purity 3-isocyanate propyltriethoxysilane and synthetic method thereof
PendingCN107759627ASimple processEasy to operateGroup 4/14 element organic compoundsChemical synthesisLiquid product
The invention discloses a synthesizer for high-purity 3-isocyanate propyltriethoxysilane and a synthetic method thereof and belongs to the field of chemical synthesis. The synthetic method disclosed by the invention comprises the following steps: heating a fixed bed reactor to 350-400 DEG C, and maintaining the temperature for later use; regulating a reducing valve to introduce protective gases into the fixed bed reactor, introducing a raw material [3-(triethoxysilicyl)propyl]ethyl carbamate by a metering pump within 3-30 minutes introducing after the protective gases, and carrying out a thermal cracking reaction for 2-4 hours; cooling the mixture reacted in the step 2 by a first condenser, cooling the first condenser by adopting hot water at the temperature of 80 DEG C, enabling the cooled mixture to enter a gas-liquid separator to be separated, and enabling the separated liquid to enter a product collection tank by virtue of a cooler. According to the method disclosed by the invention, 97.5g of the high-purity 3-isocyanate propyltriethoxysilane is obtained, the yield is 81.3%, and the gas chromatographic detection purity is 99.19%. The production process is safe, the process is simple, the product is easy to separate, and reactive raw materials are completely converted.
Owner:INST OF PETROCHEM HEILONGJIANG ACADEMY OF SCI
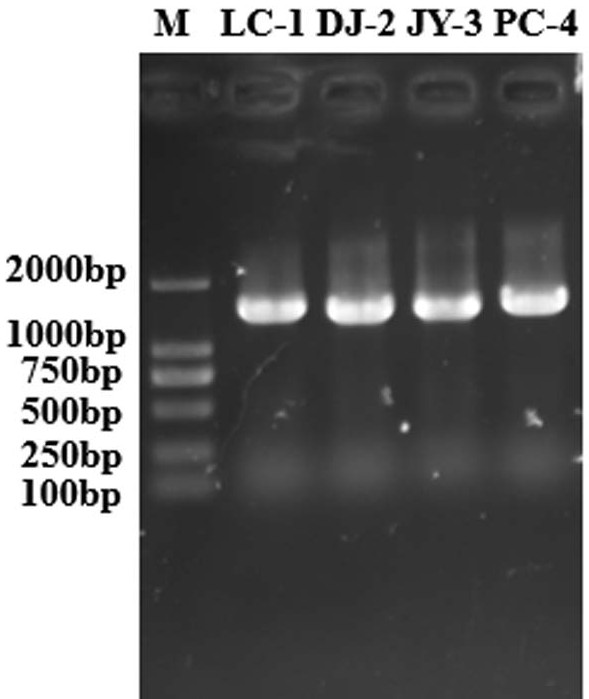
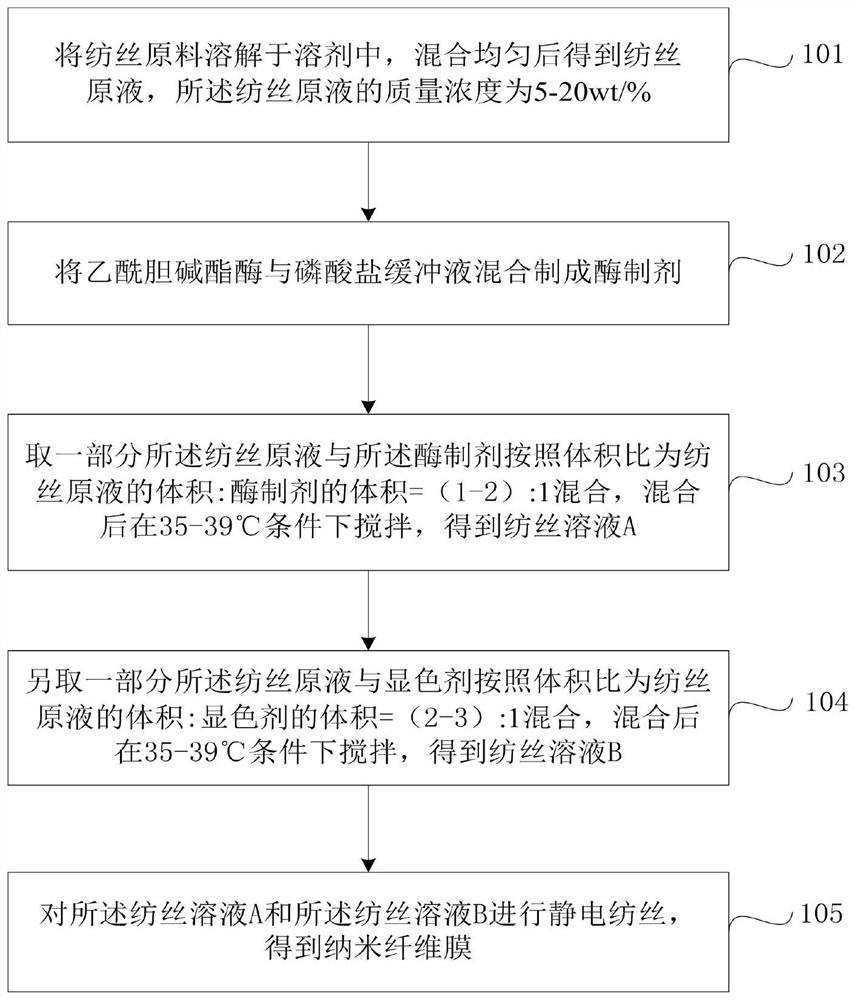
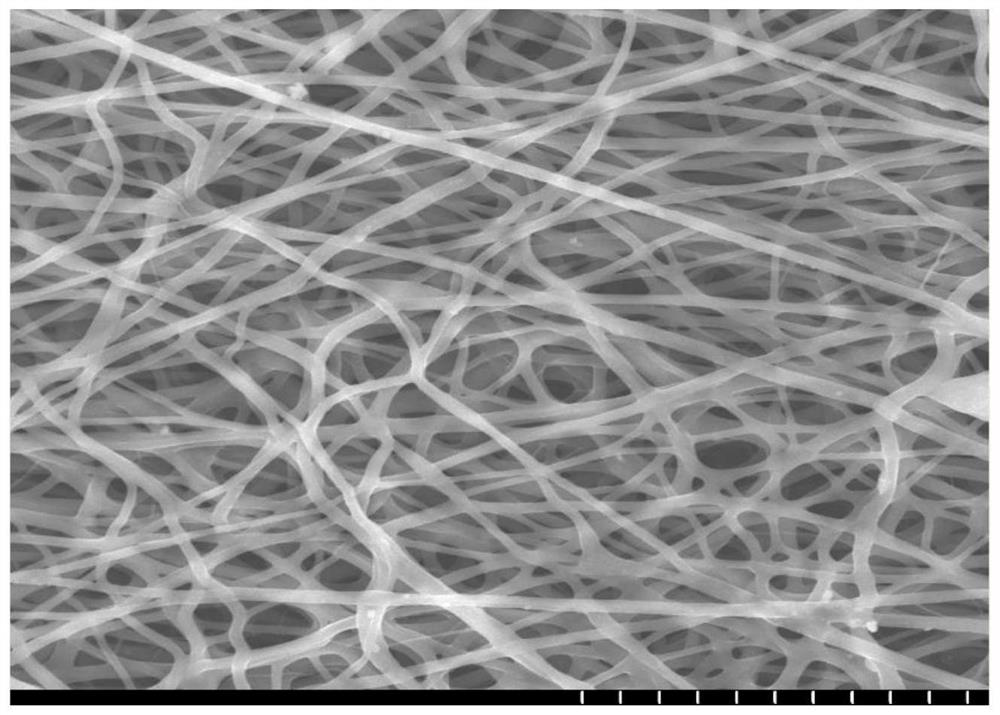
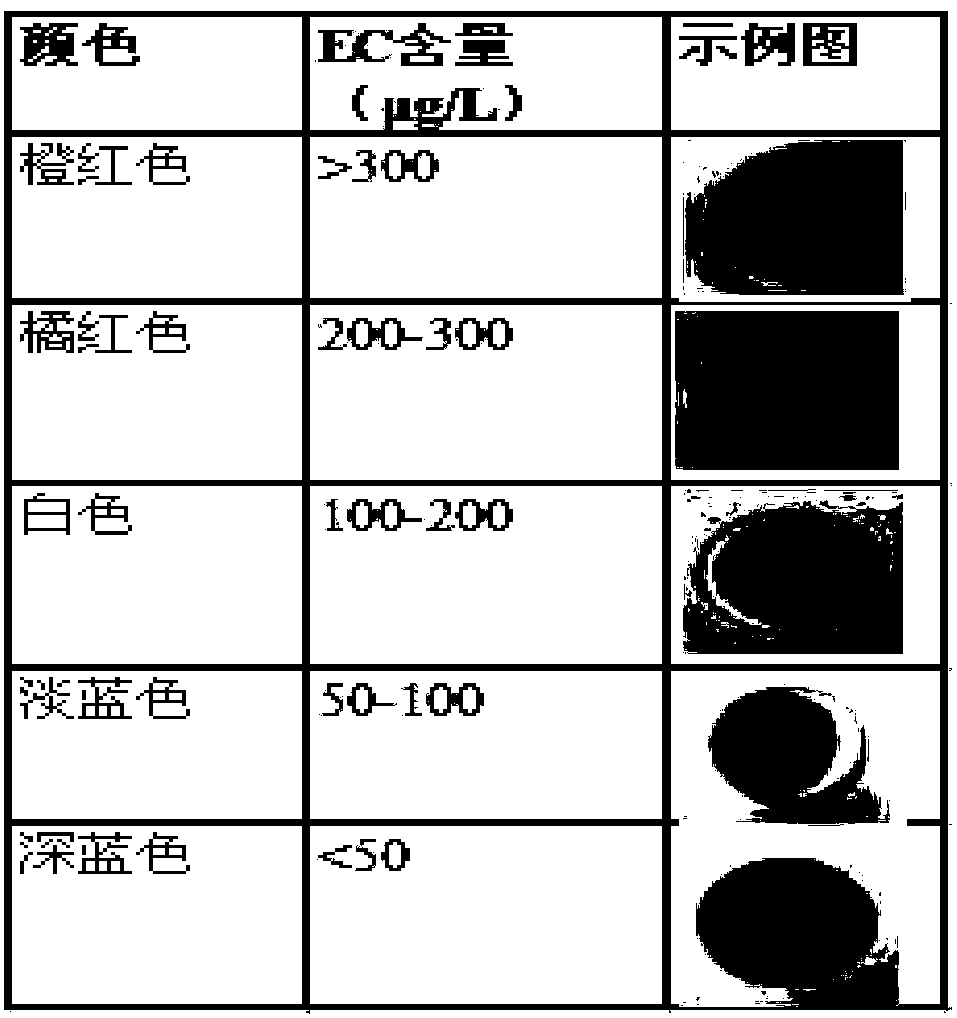
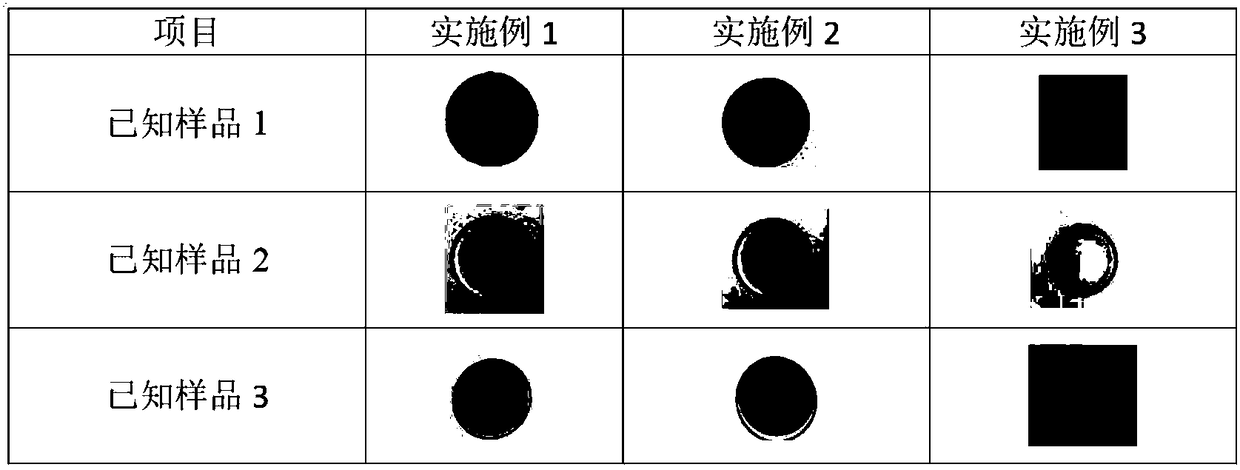
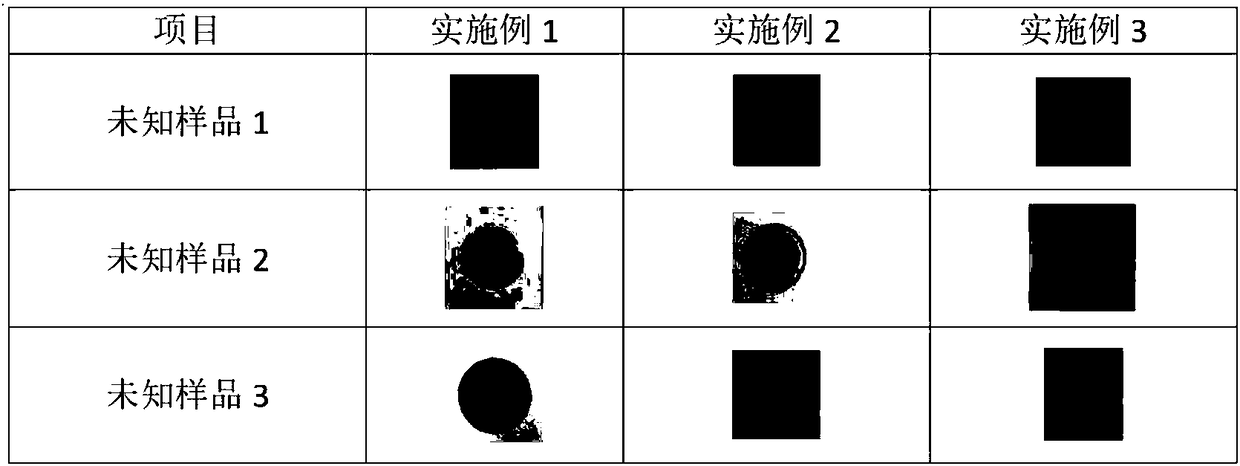
Preparation method for nanofiber membrane used for detecting ethyl carbamate
ActiveCN108505214AJudgment contentMonocomponent cellulose artificial filamentBiochemical treatment with enzymes/microorganismsCarbamatePhosphate
The invention provides a preparation method for a nanofiber membrane used for detecting ethyl carbamate. The preparation method comprises the steps that spinning materials are dissolved in solvent andmixed uniformly to obtain a spinning solution; acetylcholin esterase and a phosphate buffer are mixed to prepare an enzymic preparation; parts of spinning solution and the enzymic preparation are mixed according to the volume ratio that the spinning solution volume to the enzymic preparation volume is (1-2):1, and a spinning solution A is obtained; another parts of spinning solution and a color developing agent are mixed according to the volume ratio that the spinning solution volume to the color developing agent volume is (2-3):1, and a spinning solution B is obtained; the spinning solutionA and the spinning solution B are subjected to electrostatic spinning to obtain the nanofiber membrane. When the nanofiber membrane is utilized to detect ethyl carbamate, the contact area between theethyl carbamate and nanofibers is larger, so that the ethyl carbamate, the color developing agent and the enzymic preparation take effect, thereby generating a color developing result, and the color developing result is utilized to quickly and accurately judge the ethyl carbamate content in a sample.
Owner:南京宁高晶测生物科技有限公司
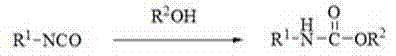
Method for preparing N-substituted ethyl carbamate
InactiveCN102372652ARaw materials are cheap and easy to getLow costCarbamic acid derivatives preparationOrganic compound preparationZinc bromideChemical synthesis
The invention discloses a method for preparing N-substituted ethyl carbamate, which belongs to the technical field of chemical synthesis. According to the invention, the method comprises the following steps of: by using tetrahydrofuran as solvent and isocyanate as raw material, reacting tetrahydrofuran and isocyanate for 8-12 hours at 40-45 DEG C under the effect of a Reformatsky reagent (zinc bromide based ethyl acetate); and extracting and purifying a reaction product so as to obtain the N-substituted ethyl carbamate. The method for preparing the N-substituted ethyl carbamate, disclosed by the invention, has the advantages of single synthesis product, high total yield (more than 70%), high purity (more than 99%), simple synthesis process, higher efficiency, mild reaction condition and friendliness to environment.
Owner:NORTHWEST NORMAL UNIVERSITY
A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds
ActiveCN113248454BEmission reductionFew reaction stepsOrganic chemistryAntiparasitic agentsVeterinary DrugsAniline
The present invention relates to the technical field of veterinary medicine, in particular to a method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2H,4H)-diketone compounds, comprising the following steps: (1 ) using dichloroacetyl chloride and ethyl carbamate as reaction raw materials, adding an acid-binding agent and a reaction solvent, heating and undergoing heat preservation reaction, distillation and rectification to obtain (2,2-dichloroacetyl) ethyl carbamate; (2) adding the aniline compound shown in structural formula 2 into hydrochloric acid, after being treated with diazotization reagent, reducing reagent, and acid precipitation reagent, and then washing with neutralization water to obtain the phenylhydrazine compound shown in structural formula 3; (3) Add phenylhydrazine compounds and (2,2-dichloroacetyl) ethyl carbamate into anhydrous acetic acid, add reaction aids, heat until the reaction is complete, and then undergo distillation to obtain 2-[phenyl]-1, 2,4‑triazine‑3,5(2H,4H)‑diones. By using the method, the overall reaction steps are shortened, the economic benefit is improved, and the discharge of three wastes is reduced.
Owner:SHANDONG GUOBANG PHARMA +1
Modified release formulation and methods of use
A modified release pharmaceutical formulation includes about 30-70% N-(2-amino-4- (fluorobenzylamino) -phenyl) carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix including hydroxypropylmethylcellulose (HPMC), about 1.0-10% of an anionic surfactant, and an enteric polymer. The pharmaceutical formulation produces a sustained plasma concentration of retigabine following administration to a subject for 4-20 hours longer than the time required for in vitro release of 80% of retigabine. A formulation includes about 30-70% N-(2-amino-4- (fluorobenzylamino) -phenyl) carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix, and an agent for retarding release in the gastric environment. The plasma concentration vs. time profile of this formulation is substantially flat over an extended period lasting for about 4 hours to about 36 hours. A method of treating a disorder characterized by nervous system hyperexcitability includes administering to a subject an effective amount of these pharmaceutical formulations.
Owner:威朗国际制药公司
Positive photosensitive resin composition, pattern forming method and application thereof
InactiveCN106200267BPhotosensitive materials for photomechanical apparatusNon-linear opticsMethacrylatePolymer science
The present invention relates to a positive-type photosensitive resin composition comprising a novolac resin (A), an esterified product (B) of an o-naphthoquinonediazide sulfonic acid, an ethyl carbamate (methyl) acrylic ester compound (C) comprising at least six (meth) acryloyl groups per molecule, and a solvent (D). The present invention also relates to a method for forming a pattern through the positive-type photosensitive resin composition and its use in a thin film transistor array substrate and a liquid crystal display element. The above-mentioned positive-type photosensitive resin composition has good pattern contrast and releasability.
Owner:CHI MEI CORP
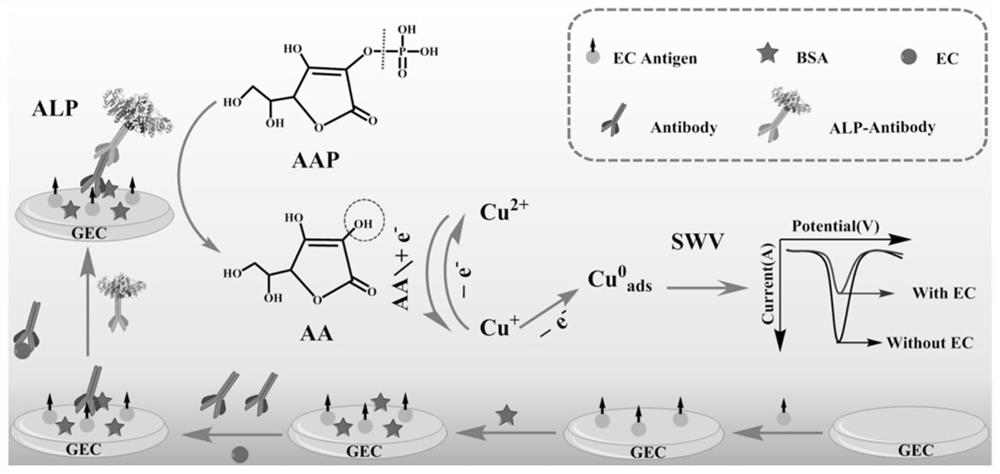
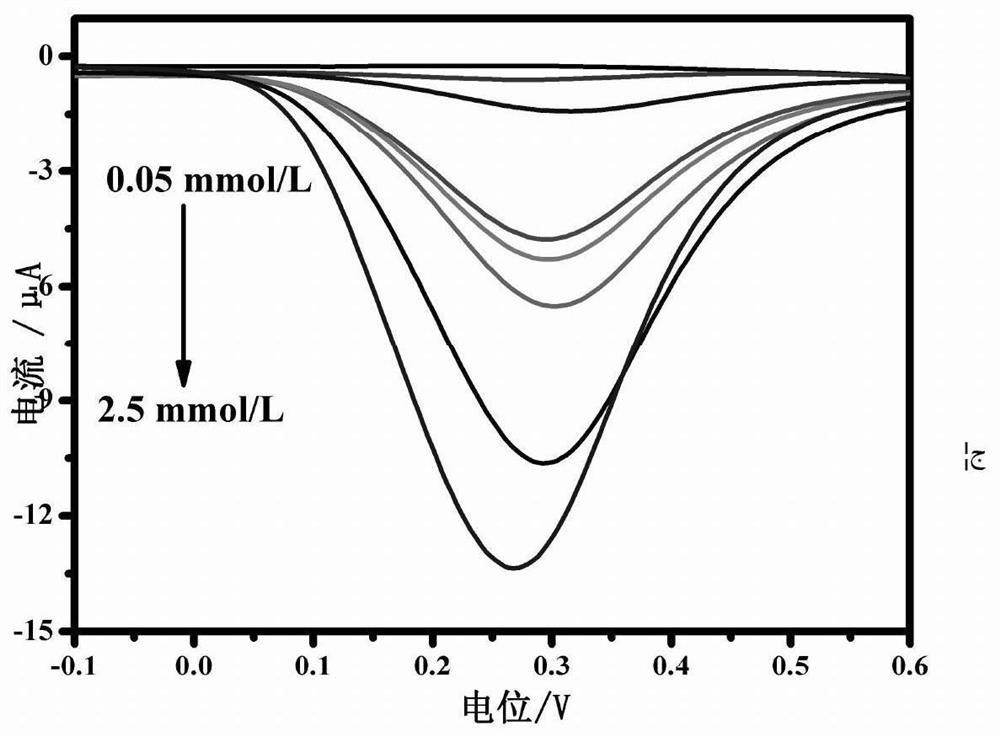
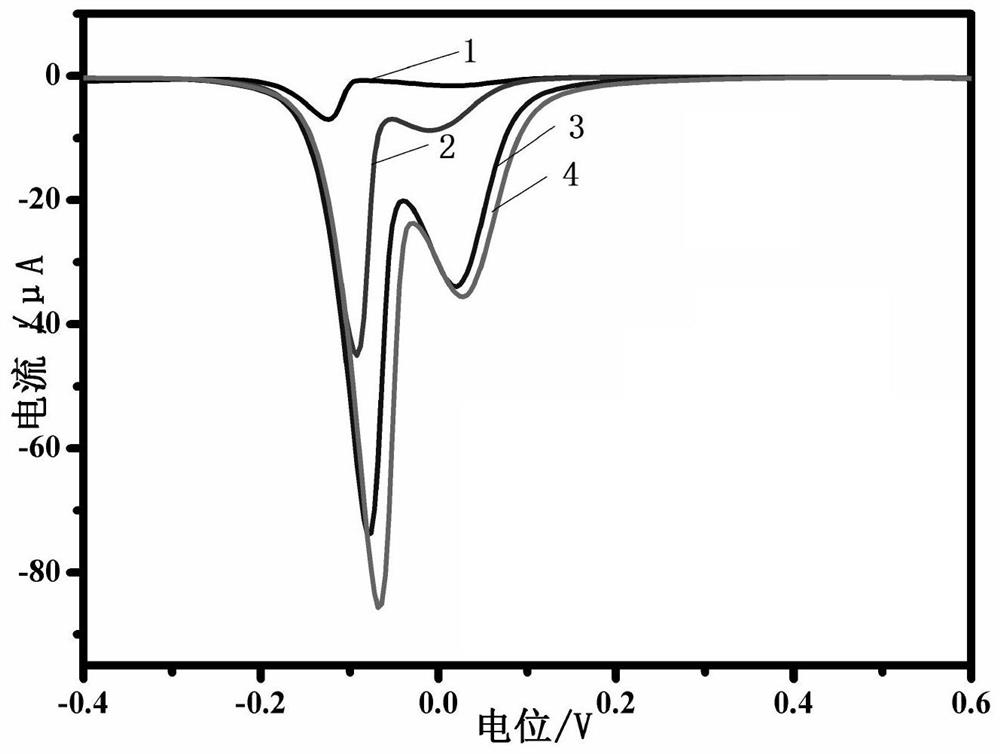
Preparation method and application of electrochemical immunosensor based on enzyme induction
PendingCN112362710AEasy to operateHigh sensitivityMaterial electrochemical variablesSquare wave voltammetryElisa method
The invention discloses a preparation method and application of an electrochemical immunosensor based on enzyme induction. The preparation method comprises the following steps of: (1) cleaning a glassy carbon electrode; and (2) constructing a probe; The construction of the probe specifically comprises the following steps of: dropwise adding an ethyl carbamate coating antigen (Ag) to the glassy carbon electrode for incubation; soaking the glassy carbon electrode in a BSA solution; soaking the glassy carbon electrode in an ethyl carbamate indirect rabbit polyclonal antibody (Ab1) for incubation;and finally, soaking the glassy carbon electrode in mouse polyclonal antibody-alkaline phosphatase (Ab2-ALP) for incubation. With the preparation method of the invention adopted, a competitive electrochemical immunosensor based on basic phosphatase (ALP) induced Cu <2+> / Cu<+> conversion is developed. The competitive electrochemical immunosensor can be used for sensitively detecting ethyl carbamate. Compared with the detection sensitivity of a traditional enzyme-linked immunosorbent assay, the detection sensitivity of the competitive electrochemical immunosensor is improved through three rounds of signal amplification, namely catalytic reaction of ALP enzyme, Cu<2+> / Cu<+> conversion and square wave voltammetry signal output.
Owner:HUBEI UNIV OF TECH
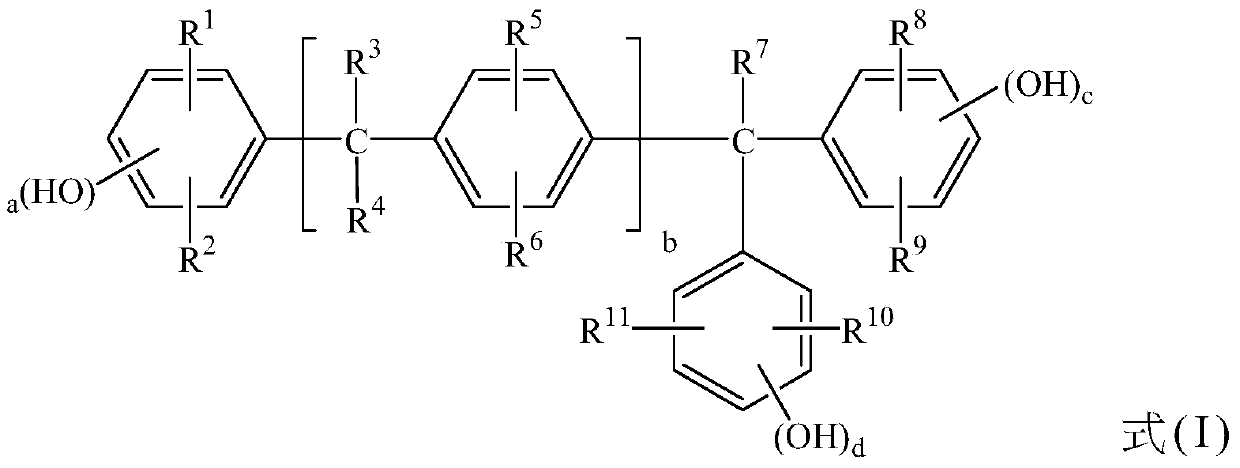
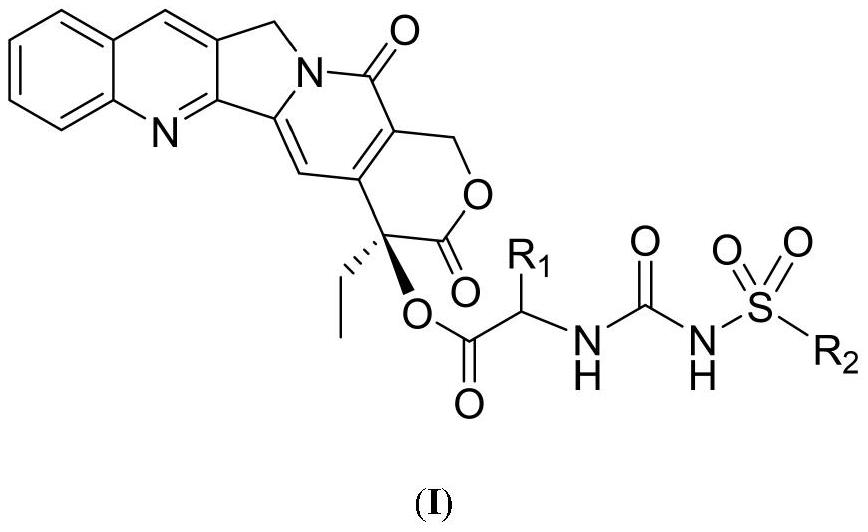
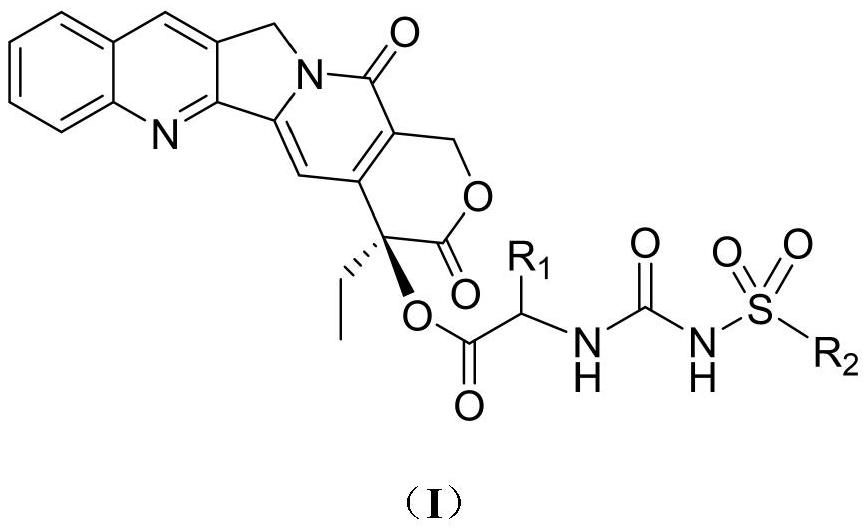
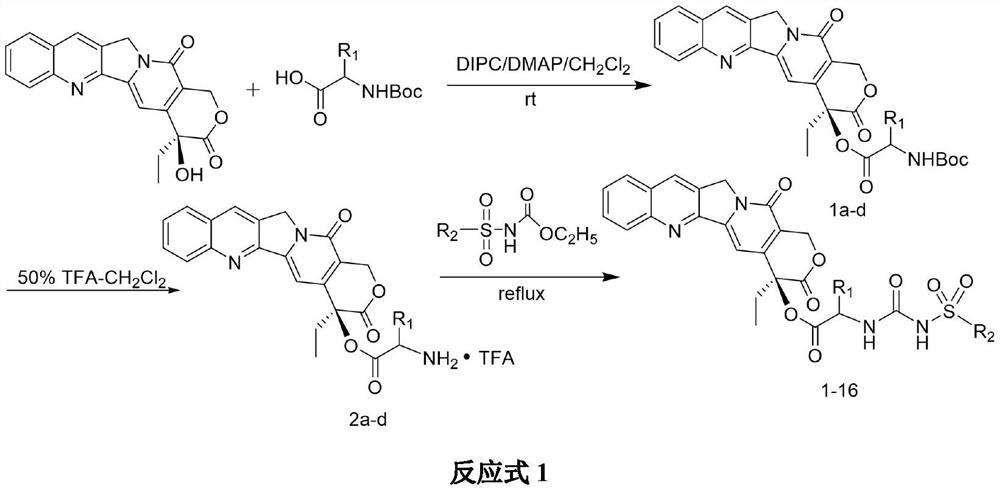
Camptothecin 20-site modified sulfonylurea compound as well as preparation method and application thereof
InactiveCN111689977AHigh purityNovel structureOrganic chemistryAntineoplastic agentsStructural formulaCarbamic acid ethyl ester
The invention relates to a camptothecin 20-site modified sulfonylurea compound shown as a formula (I), a preparation method of the compound, and application of the compound in preparation of antitumordrugs. The chemical general formula of the compound is shown as a structural formula (I). The preparation method of the compound in the formula (I) is characterized in that different 20-amino acid-camptothecin trifluoroacetate reacts with different substituted sulfonyl ethyl carbamate to obtain the compound. Cytotoxic activity test proves that the compounds have good cytotoxic activity, the antitumor activity of most of the compounds is higher than that of a clinical control drug irinotecan, the antitumor activity of part of the compounds is equivalent to that of a clinical control drug topotecan, and the compounds can be used for preparing antitumor drugs. The preparation method is simple, raw materials are cheap and easy to obtain, and product purity is high.
Owner:LANZHOU UNIVERSITY
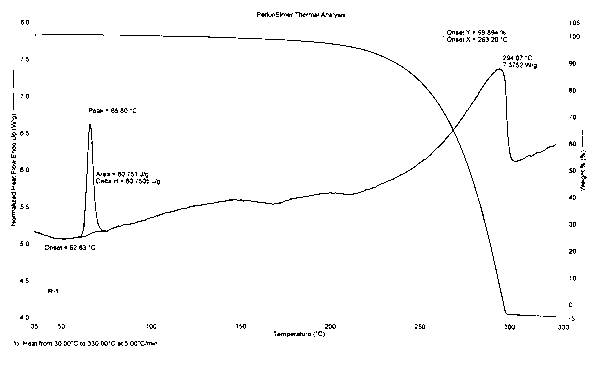
Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid
InactiveUS9149468B2Fast dissolving actionImprove bioavailabilityBiocideNervous disorderBULK ACTIVE INGREDIENTCarbamic acid ethyl ester
The invention relates to novel multicomponent crystals, to the production thereof, and to the use thereof for treating pain conditions, in particular of unclear genesis, by means of a simultaneous effect on pains which are caused by muscle tension or degenerative joint diseases as well as on pains that are based on inflammatory processes. The novel multicomponent crystals contain ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester (flupirtine) and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid (diclofenac) as the sole active ingredient combination and can be produced by dissolving the two components in a molar ratio of 1.0:0.9 to 1.0:1.1 in an inert organic solvent and subsequently crystallizing the complex compound.
Owner:TEVA
Modified release formulation and methods of use
A modified release pharmaceutical formulation includes about 30-70% N-(2-amino-4-(fluorobenzylamino)-phenyl) carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix including hydroxypropylmethylcellulose (HPMC), and an enteric polymer. The pharmaceutical formulation produces a sustained plasma concentration of retigabine following administration to a subject for 4-20 hours longer than the time required for in vitro release of 80% of retigabine. The plasma concentration vs. time profile of this formulation is substantially flat over an extended period lasting for about 4 hours to about 36 hours. A method of treating a disorder characterized by nervous system hyperexcitability includes administering to a subject an effective amount of these pharmaceutical formulations.
Owner:VALEANT PHARMA INT
Modified release formulation and methods of use
A modified release pharmaceutical formulation includes about 30-70% N-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix including hydroxypropylmethylcellulose (HPMC), about 1.0-10% of an anionic surfactant, and an enteric polymer. The pharmaceutical formulation produces a sustained plasma concentration of retigabine following administration to a subject for 4-20 hours longer than the time required for in vitro release of 80% of retigabine. A formulation includes about 30-70% N-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ethyl ester (retigabine), or a pharmaceutically acceptable salt, solvate or hydrate thereof, about 5-30% of a drug delivery matrix, and an agent for retarding release in the gastric environment. The plasma concentration vs. time profile of this formulation is substantially flat over an extended period lasting for about 4 hours to about 36 hours. A method of treating a disorder characterized by nervous system hyperexcitability includes administering to a subject an effective amount of these pharmaceutical formulations.
Owner:VALEANT INT PHARM
Degenerate primer for quickly screening citrulline degrading bacteria and application of degenerate primer
PendingCN113584137AImprove versatilityImprove work efficiencyMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyBase J
The invention discloses a degenerate primer for quickly screening citrulline degrading bacteria. The sequence of the degenerate primer is as follows: PTP1-F: 5 '-NACHACHGGCCAHACRAA-3'; and PTP2-R: 5 '-GTHGAYATHATGGTMTTYAT-3'; wherein the degenerate base code Y is equal to C / T, R is equal to A / G, H is equal to A / C / T, B is equal to G / T / C, and N is equal to A / G / C / T. The invention also discloses an application of the designed degenerate primer in screening citrulline degrading bacteria. A pair of degenerate primers is designed according to the PTP gene with citrulline utilization capacity for the first time, the degenerate primers are universal primers with a screening function, a specific probe primer does not need to be designed for a certain strain, and the degenerate primers are convenient and fast to use, good in universality and high in working efficiency and have good screening and recognition functions; a strain guarantee is provided for subsequently exploring the difference of citrulline utilization capacity of PTP genes of different strain sources and comparing the influence of environmental factors, and a new solution idea and method are provided for reducing or eliminating ethyl carbamate generated in the fermentation process.
Owner:HENAN AGRICULTURAL UNIVERSITY
A kind of preparation method of nanofiber membrane for detecting urethane content
ActiveCN108505214BJudgment contentMonocomponent cellulose artificial filamentBiochemical treatment with enzymes/microorganismsPolymer scienceCarbamate
The invention provides a preparation method for a nanofiber membrane used for detecting ethyl carbamate. The preparation method comprises the steps that spinning materials are dissolved in solvent andmixed uniformly to obtain a spinning solution; acetylcholin esterase and a phosphate buffer are mixed to prepare an enzymic preparation; parts of spinning solution and the enzymic preparation are mixed according to the volume ratio that the spinning solution volume to the enzymic preparation volume is (1-2):1, and a spinning solution A is obtained; another parts of spinning solution and a color developing agent are mixed according to the volume ratio that the spinning solution volume to the color developing agent volume is (2-3):1, and a spinning solution B is obtained; the spinning solutionA and the spinning solution B are subjected to electrostatic spinning to obtain the nanofiber membrane. When the nanofiber membrane is utilized to detect ethyl carbamate, the contact area between theethyl carbamate and nanofibers is larger, so that the ethyl carbamate, the color developing agent and the enzymic preparation take effect, thereby generating a color developing result, and the color developing result is utilized to quickly and accurately judge the ethyl carbamate content in a sample.
Owner:南京宁高晶测生物科技有限公司
Methyl carbamate refining method
PendingCN114656375AReasonable workmanshipEasy to operateCarbamic acid derivatives preparationOrganic compound preparationMethyl carbamateEthyl ester
According to the methyl carbamate refining method, methyl carbamate is subjected to recrystallization separation and vacuum drying treatment through methylbenzene under nitrogen protection, ethyl carbamate is separated out, and a refined methyl carbamate product is obtained. The methyl carbamate is recrystallized and purified for multiple times by adopting methylbenzene, so that the ethyl carbamate in the methyl carbamate is separated out, the impurity of the ethyl carbamate is reduced to a level which cannot be detected, and the method has the advantages of reasonable process, convenience in operation and high purity.
Owner:山东禹城易澳科技有限公司
Novel crystal form D of Retigabine and preparation method thereof
ActiveCN102531966BImprove securityLarge particle sizeNervous disorderCarbamic acid derivatives preparationPhysical chemistryPharmaceutical drug
The invention relates to the field of medicinal chemistry, in particular to a novel crystal form D of N-(2- amino-4-(4- fluoro benzyl amino) phenyl) ethyl carbamate (Retigabine) and a preparation method of the novel crystal form D. The invention provides X-ray powder diffraction characteristic absorption peaks and DSC (differential scanning calorimetry) endothermic transition peaks of the novel crystal form. The novel crystal form D of the Retigabine is characterized in that the crystal has characteristic absorption peaks at angles of (2 theta) 5.18 DEG, 10.60 DEG, 13.22 DEG, 13.98 DEG, 15.70DEG, 18.26 DEG, 20.46 DEG, 21.40 DEG, 22.22 DEG, 22.58 DEG, 23.06 DEG, 24.44 DEG, 24.82 DEG, 27.38 DEG and 28.28 DEG under the X-ray powder diffraction and the DSC endothermic transition of the novelcrystal form is at a temperature of 60-70 DEG C. The novel crystal form D of the Retigabine is thin in grain size (the average grain size is 20-35 mum and normal distributed), and can be used for medicinal preparation production with no need for physical smashing, therefore, the production link is decreased, the production cost is reduced, the pollution and the medicinal property changing caused in smash process are avoided and the clinical medication safety is increased.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Ethyl carbamate hydrolase mutants with improved thermostability
ActiveCN105420210BPromote degradationImprove thermal stabilityHydrolasesAlcoholic beverage preparationEthyl esterGene engineering
The invention discloses ethyl carbamate hydrolytic enzyme mutants capable of improving the thermostability, and belongs to the technical field of gene engineering and enzyme engineering. By means of a molecular technical means, the ethyl carbamate hydrolytic enzyme mutants capable of improving the thermostability are obtained, and the half-life periods of the ethyl carbamate hydrolytic enzyme mutants Q328C and Q328V are increased by 7.46 times and 1.96 times respectively compared with a native enzyme. The mutants Q328C, Q328R and Q328 V all have better tolerance at 30 DEG C or above than the native enzyme. In addition, the tolerance to ethyl alcohol and the tolerance to acid of the mutant Q328C are improved.
Owner:JIANGNAN UNIV
Preparation method and application of nano-modified PTFE and polyester composite film for anti-icing of wind turbine blades
ActiveCN106313811BSolving non-adhesive technical problemsConvenient engineering constructionSynthetic resin layered productsLaminationMeth-Polymer science
The invention provides a preparation method and application of a nano-modified PTFE and polyester-based composite film for preventing fan blades from icing. The method includes the steps of PTFE film modification, lamination complexing and photo-crosslinked adhesive application. A modifier is prepared from antimony-doped tin oxide nano-crystals, nano-titanium dioxide, nano-silicon carbide, an organic fluorine waterproofing agent and pentaerythritol tri-(3-aziridinyl)-propionate; in lamination complexing, a bonding complexing agent is prepared from 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate, vinyl acetate, ethyl carbamate, alpha-linolenic acid, (2)ethoxylated bisphenol A dimethacrylate, trimethylolpropane triacrylate and benzoyl peroxide; a photo-crosslinked adhesive is prepared from a poly[butyl acrylate-glycidyl methacrylate-n-butoxy methacrylamide]copolymer, vinyl acetate, butyl acrylate, an acrylate derivative, a photoinitiator and dimethylformamide. The method and the composite film solve the non-adhesion problem that a PTFE film can not be pasted on the surfaces of fan blades with an adhesive directly.
Owner:NANJING HAOHUI HI TECH CO LTD
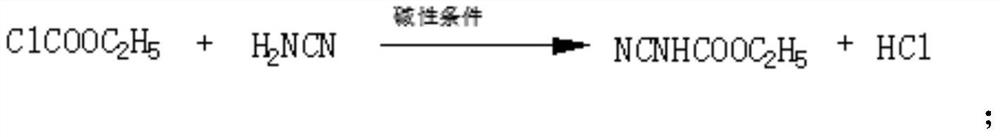
Method for synthesizing ethyl N-methyl cyanocarbamate by methylation reagent
InactiveCN111825571AOvercome the problem of wastewater acid back corrosion equipmentSmooth responseOrganic chemistryEthyl chloroformateEthyl ester
The invention discloses a method for synthesizing ethyl N-methyl cyanocarbamate by using a methylation reagent. The method comprises the following steps of: 1) reacting cyanamide with ethyl chloroformate under a strong alkali condition to generate ethyl cyanocarbamate; and 2) carrying out reaction on ethyl cyanocarbamate and a methylation reagent dimethyl carbonate under a strong alkaline condition to generate ethyl N-methyl cyanocarbamate. According to the new method for synthesizing the ethyl N-methyl cyanocarbamate by using the methylation reagent, the low-toxicity and safe methylation reagent dimethyl carbonate is used for replacing dimethyl sulfate; the method is safer, can overcome the problem of equipment corrosion caused by acid regurgitation of wastewater in the conventional method, and has the advantages of more stable reaction, few byproducts, higher yield and stronger operability.
Owner:XINYI AGRI CHEM PLANT JIANGSU PROV
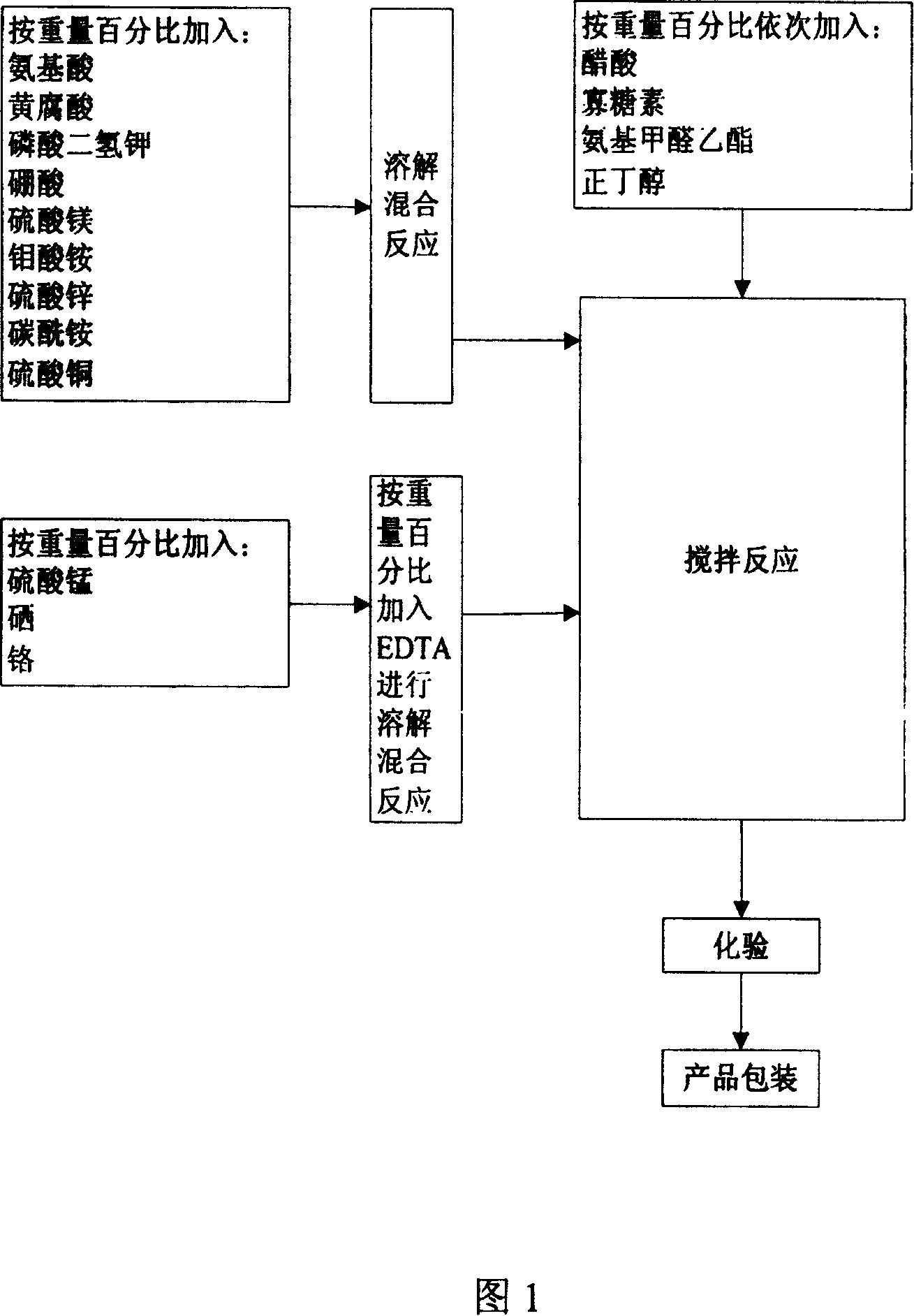
Oligo-acid fertilizer liquid and its preparation method
InactiveCN100339006CAvoid BactericidalPromote growth and developmentBiocidePlant growth regulatorsEthylic acidMonopotassium phosphate
Disclosed is an oligo-acid fertilizer liquid and its preparation method, where the constituents include organelle 0.2-0.5%, ethyl carbamate 0.2-1%, amino acid 1-10%, fulvic acid 2-10%, carbamide 5-15%, potassium dihydeogenorthophosphate 6-18%, magnesium sulfate 8-35%, boracic acid 5-15%, zinc sulfate 8-24%, ammonium molybdate 0.2-1%, copper sulfate 4-12%, manganese sulfate 4-12%, selenium 1-3%, chromium 1-3%, EDTA 0.3-1%, and acetic acid 0.2-1%.
Owner:四川慧绿中医科技股份有限公司
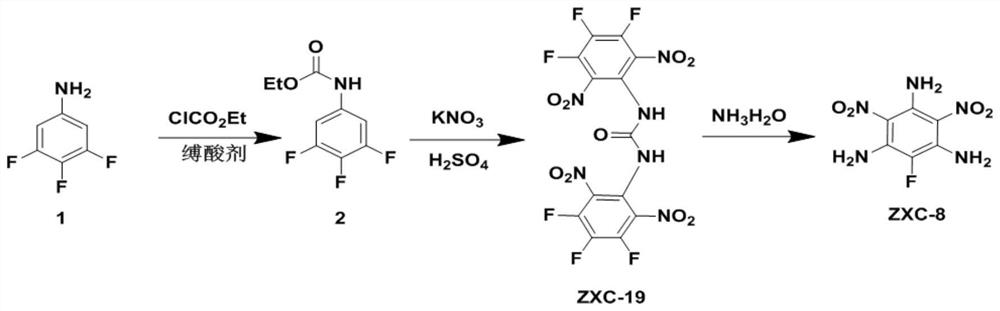
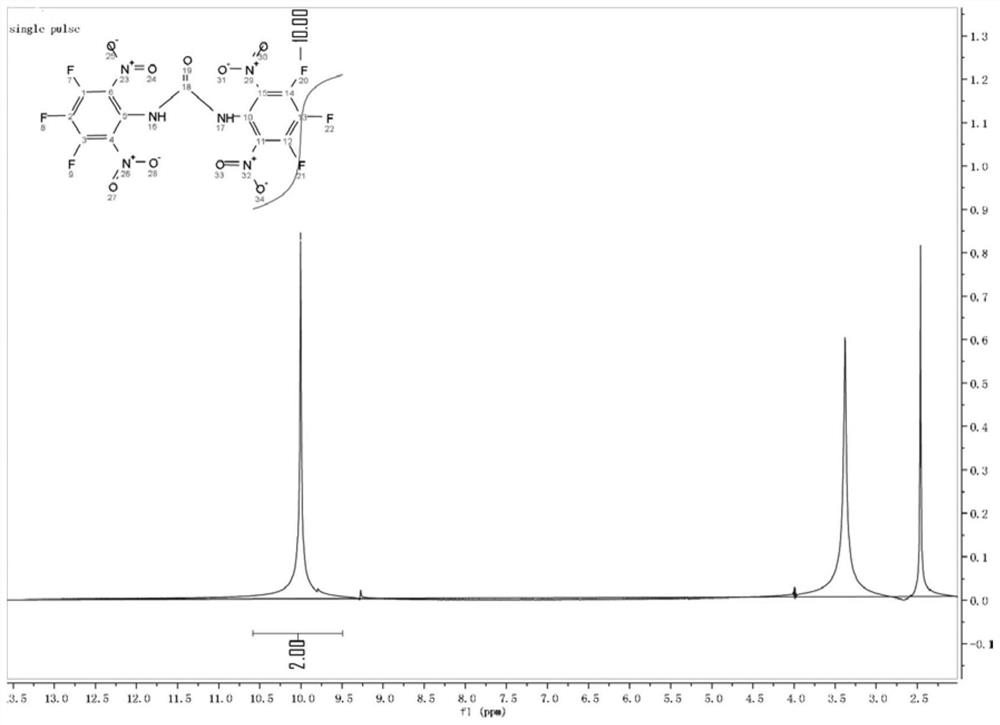
Energetic material 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl)urea and its preparation method and application
ActiveCN110218164BImprove performanceImprove detonation performanceUrea derivatives preparationOrganic compound preparationOrganic solventNitration
The structural formula of 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl) urea of the present invention comprises the following steps for its preparation method: 3,4,5-trifluoroaniline is substituted The reaction obtains the intermediate product (3,4,5-trifluorophenyl) ethyl carbamate, and then through nitration reaction to obtain 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl ) urea. ZXC‑19 has poor solubility in water and is soluble in most organic solvents. It has a very high density at 298K, has good thermal stability, high solid-state heat of formation and good detonation performance, low sensitivity, and no combustion products. Small molecular weight, suitable for use as rocket propellant. 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl) urea has a very simple synthesis method, high yield, easy industrial production, environmental friendliness, and easy recrystallization. An important raw material for ZXC‑8.
Owner:XINYANG NORMAL UNIVERSITY
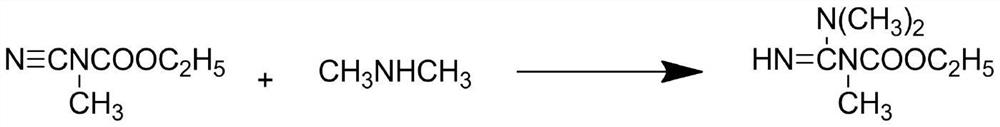
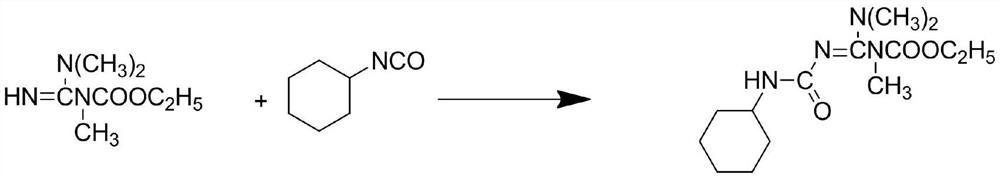
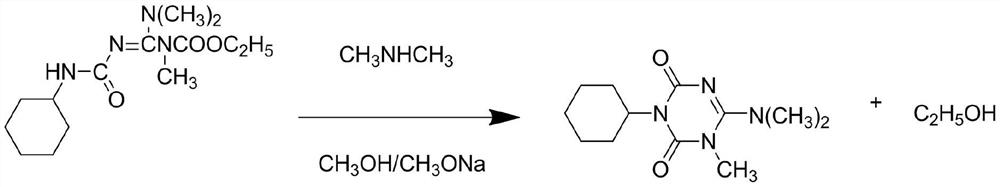
A kind of production technology that improves hexazinone yield
The invention discloses a production process for improving the yield of hexazinone, comprising the following steps: step S1, first using cyanamide and ethyl chloroformate to generate ethyl cyanocarbamate, and then combining ethyl cyanocarbamate with dimethyl sulfate The ester synthesizes a methyl product under the catalytic condition of a catalyst; step S2, synthesizes a guanidine derivative with a methyl product and dimethylamine; step S3, uses chloroform to perform multiple extractions on the guanidine derivative; step S4, performs precipitation on the guanidine derivative Processing: step S5, reacting guanidine derivatives with cyclohexyl isocyanate to synthesize a hexazinone intermediate under the condition of toluene as a solvent; step S6, performing a cyclization reaction between the hexazinone intermediate and sodium methoxide to prepare hexazinone and washing with water; Step S7, purifying and drying hexazinone to obtain the technical product of hexazinone; the production process of the present invention is easy for industrial production and has strong practicability, and solves the technical problem of low yield of the technical product of hexazinone in the prior art.
Owner:ANHUI GUANGXIN AGROCHEM
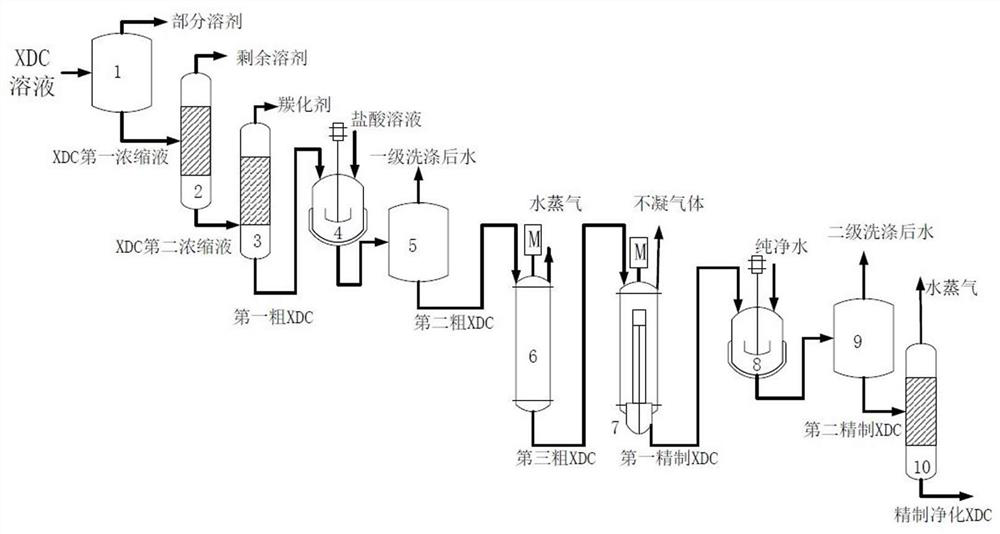
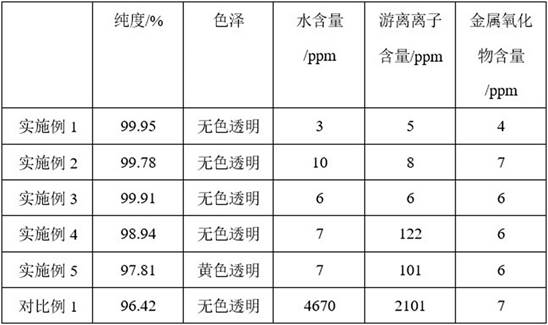
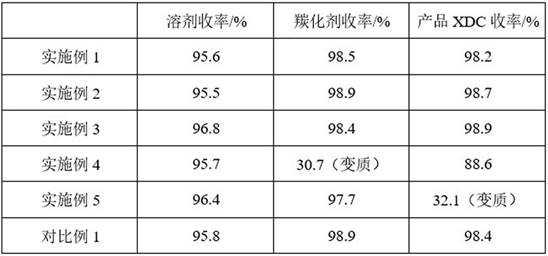
Method and device for separating, refining and purifying intermediate m-xylylene dicarbamate solution and application of intermediate m-xylylene dicarbamate solution
ActiveCN114478321ARealize separation, refinement and purificationRemove completelyCarbamic acid derivatives preparationOrganic compound preparationXylyleneImpurity ions
The invention provides a method and a device for separating, refining and purifying an intermediate ethyl m-xylylene dicarbamate solution and application of the intermediate ethyl m-xylylene dicarbamate solution. The method comprises the following steps: sequentially carrying out flash evaporation, first rectification and second rectification on an XDC solution, carrying out first-stage washing, first-stage oil-water separation, first-stage evaporation, second-stage evaporation, second-stage washing, second-stage oil-water separation and dehydration treatment to obtain refined and purified XDC; according to the method, a series of separation processes are designed according to the properties of the XDC and impurities contained in the solution, so that the separation, refining and purification of the XDC can be safely and stably realized, the impurities in the XDC solution are thoroughly removed, the residual quantity of impurity ions and metal oxides in the product is less than 10ppm, and the product quality is improved; the method can also efficiently recover the carbonylation agent and the solvent for cyclic utilization, has certain economical efficiency, and is beneficial to industrial application.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
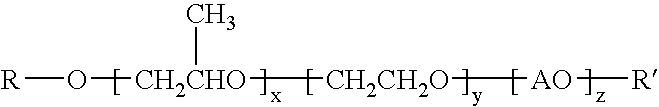
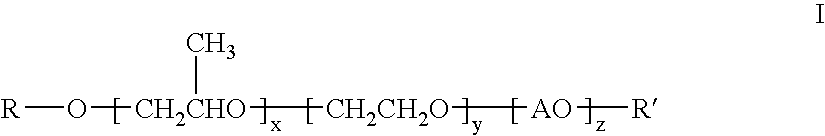
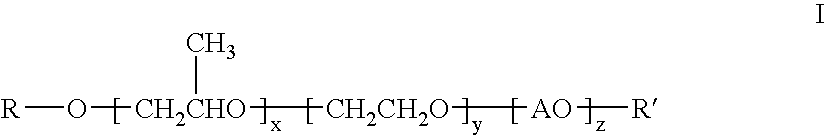
![A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds](https://images-eureka.patsnap.com/patent_img/1a53d897-21b5-4b78-a8fc-ab574cd77cfa/DEST_PATH_IMAGE005.png)
![A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds](https://images-eureka.patsnap.com/patent_img/1a53d897-21b5-4b78-a8fc-ab574cd77cfa/DEST_PATH_IMAGE007.png)
![A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds A method for preparing 2-[phenyl]-1,2,4-triazine-3,5(2h,4h)-dione compounds](https://images-eureka.patsnap.com/patent_img/1a53d897-21b5-4b78-a8fc-ab574cd77cfa/DEST_PATH_IMAGE009.png)
![Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid](https://images-eureka.patsnap.com/patent_img/582ed117-f164-4c65-b64c-4cf73812de9c/US09149468-20151006-D00001.PNG)
![Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid](https://images-eureka.patsnap.com/patent_img/582ed117-f164-4c65-b64c-4cf73812de9c/US09149468-20151006-D00002.PNG)
![Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid Multicomponent crystals made ([2-amino-6-(4-fluoro-benzylamino)-pyridin-3-yl]-carbamic acid ethyl ester and 2-[2-[(2,6-dichlorphenyl)-amino]-phenyl]-acetic acid](https://images-eureka.patsnap.com/patent_img/582ed117-f164-4c65-b64c-4cf73812de9c/US09149468-20151006-D00003.PNG)