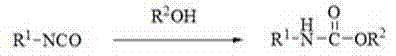
Method for preparing N-substituted ethyl carbamate
A technology of ethyl carbamate and ethyl acetate, which is applied in the preparation of N-substituted ethyl carbamate and the field of preparation of N-substituted ethyl carbamate, which can solve the lack of structure of dimethyl carbonate and urea compounds Diversity, not suitable for large-scale production, low yield of target products, etc., to achieve the effect of cheap raw materials, low cost, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment one, the preparation of ethyl phenylcarbamate
[0042] (1) Preparation of Reformatsky reagent (ethyl zinc bromide acetate): Add zinc powder (0.50 g, 7.69 mmol) and tetrahydrofuran (5 mL) into a 50 mL two-neck flask, add 1, 2-Dibromoethane (0.01 mL), heated until the solution boiled slightly, cooled naturally to room temperature, added trimethylchlorosilane (0.01 mL), stirred for 15 minutes, added ethyl bromoacetate (1.26 g, 7.57 mmol) , reacted at 45°C for 10 hours under the protection of nitrogen, the zinc powder reacted completely, and the Reformazky reagent was obtained with a yield of more than 95%.
[0043] (2) Preparation of ethyl phenylcarbamate: Add phenylisocyanate (0.30 g, 2.48 mmol) and tetrahydrofuran (5 mL) into a 50 mL two-neck flask, then slowly add ethyl zinc bromide acetate (2.48 mmol), reacted at 40°C for 8 hours under the protection of nitrogen, TLC detected the disappearance of the raw materials, quenched the reaction with saturated ammo...
Embodiment 2
[0046] Embodiment two, the preparation of ethyl 3-nitrophenylcarbamate
[0047] (1) Preparation of Reformatzky reagent (ethyl zinc bromide acetate): same as Example 1.
[0048] (2) Preparation of ethyl 3-nitrophenylcarbamate: Add 3-nitrophenylisocyanate (0.41 g, 2.48 mmol) and tetrahydrofuran (5 mL) into a 50 mL two-necked flask, then slowly add bromine dropwise Zinc ethyl acetate (4.96 mmol) was reacted at 40°C for 8 hours under the protection of nitrogen. TLC detected that the starting material disappeared, and the reaction was quenched with saturated ammonium chloride solution (5 mL), and the organic phase was extracted with diethyl ether (3×40 mL). Dry the organic solvent with anhydrous magnesium sulfate, filter, evaporate the solvent to dryness, and perform column chromatography (ethyl acetate:petroleum ether=1:5) to obtain a light yellow solid (0.40 g, 1.91 mmol) which is the target product with a yield of 77 %. Melting point: 68-70°C.
[0049] Its various performan...
Embodiment 3
[0051] Embodiment three, the preparation of ethyl 4-chlorophenylcarbamate
[0052] (1) Preparation of Reformatzky reagent (ethyl zinc bromide acetate): same as Example 1.
[0053] (2) Preparation of ethyl 4-chlorophenylcarbamate: Add 4-chlorophenylisocyanate (0.38 g, 2.48 mmol) and tetrahydrofuran (5 mL) into a 50 mL two-necked flask, then slowly add zinc bromide dropwise Ethyl acetate (7.20 mmol), reacted at 45°C for 10 hours under nitrogen protection, TLC detected the disappearance of the starting material, quenched the reaction with saturated ammonium chloride solution (5 mL), extracted the organic phase with diethyl ether (3×40 mL), and used The organic solvent was dried with anhydrous magnesium sulfate, filtered, and evaporated to dryness. After column chromatography (ethyl acetate: petroleum ether = 1:5), the target product was obtained as a white solid (0.35 g, 1.74 mmol), with a yield of 70%. Melting point: 71-73°C.
[0054] Its various performance indicators or ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com