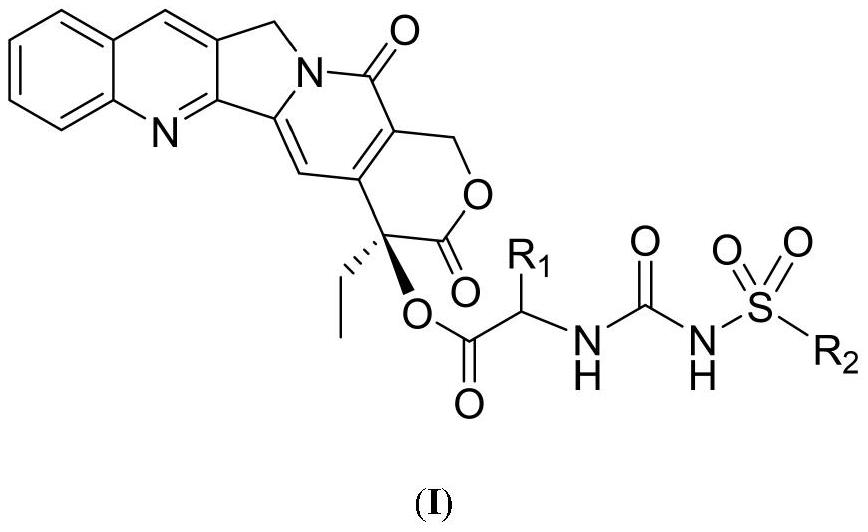
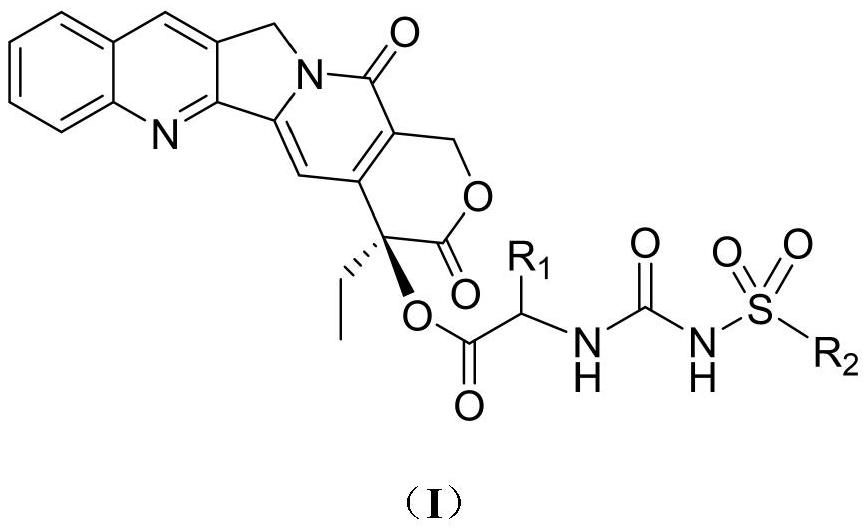
Camptothecin 20-site modified sulfonylurea compound as well as preparation method and application thereof
A technology for alkali sulfonylureas and compounds is applied in the field of preparing anti-tumor drugs, which can solve problems such as decreased anti-tumor activity, and achieve the effects of strong inhibitory effect, high product purity and simple synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the synthesis of target compound 1
[0015]
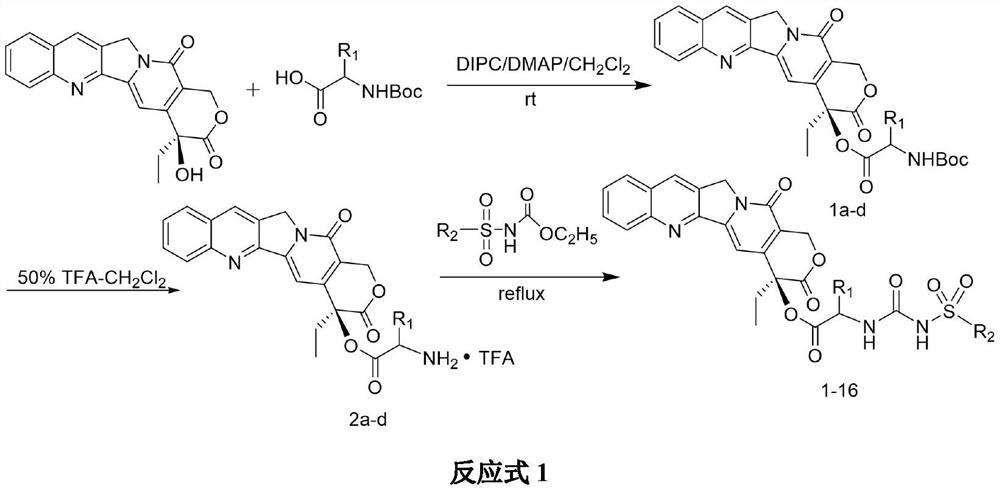
[0016] The synthesis of compound 1 is carried out according to the following chemical reaction formula 2:
[0017]
[0018] Synthesis of intermediate 1a: Dissolve N-Boc-glycine (6mmol) in dry dichloromethane, add camptothecin (3mmol), N,N'-diisopropylcarbodiethylene Amine (DIPC) (9 mmol) and 4-N,N-dimethylaminopyridine (DMAP) (6 mmol). After reacting at 0°C for about 0.5h, remove the ice bath and react overnight at room temperature. TCL detection, after the reaction is completed, filter, the reaction mixture is washed with 6% sodium bicarbonate solution, water, and saturated sodium chloride solution in turn, then dried with anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and passed through the column to obtain the yield Intermediate Compound 1a.
[0019] Synthesis of intermediate 2a: Dissolve the synthesized intermediate 1a (3 mmol) in 10 mL of dry dichloromethane, then slowly ...
Embodiment 2
[0021] Embodiment 2: the synthesis of target compound 2
[0022]
[0023] The experimental procedure is the same as in Example 1, except that ethyl methanesulfonylcarbamate is replaced by n-butylsulfonylcarbamate. The detection data of the product are as follows: Yield: 38%; Melting point: 197-198°C; 1 HNMR (DMSO-d 6 ,400MHz)δ:10.54(s,1H,SO 2 NH),8.70(s,1H,C7-H),8.15(m,2H,C12-H,C9-H),7.88(t,1H,J=6.8Hz,C10-H),7.73(t,1H ,J=7.2Hz,C11-H),7.22(s,1H,C14-H),6.92(br,1H,L-glycine- NH ),5.51(s,2H,C17-H),5.31(s,2H,C5-H),4.16(dd,1H,J=4.8,17.6Hz,C23-H),4.01(dd,1H,J= 5.2,18.0Hz,C23-H),3.17(m,2H,- CH 2 CH 2 CH 2 CH 3 ),2.09-2.15(m,2H,C18-H),1.52-1.55(m,2H,-CH 2 CH 2 CH 2 CH 3 ),1.15-1.2(m,2H,-CH 2 CH 2 CH 2 CH 3 ),0.93(t,3H,J=7.6Hz,-CH 2 CH 2 CH 2 CH 3 ),0.68(t,3H,J=7.2Hz,C19-H);Anal.Calcd For C 27 h 28 N 4 o8 S: C, 57.03; H, 4.96; N, 9.85. Found: C, 56.97; H, 5.02; N, 9.74. MS-ESI m / z: 591.1 [M+Na] + .
Embodiment 3
[0024] Embodiment 3: the synthesis of target compound 3
[0025]
[0026] The experimental procedure is the same as in Example 1, only ethyl benzenesulfonylcarbamate is used instead of ethyl methylsulfonylcarbamate. The detection data of the product are as follows: Yield: 40%; Melting point: 173-174°C; 1 H NMR (DMSO-d 6 ,400MHz)δ:11.08(s,1H,SO 2 NH ),8.71(s,1H,C7-H),8.00-8.17(m,2H,C9-H,C12-H),7.45-7.90(m,7H,C10-H,C11-H,Ph-H) ,7.16(s,1H,C14-H),6.96(br,1H,L-glycine- NH ),5.48(s,2H,C17-H),5.31(s,2H,C5-H),4.01(m,2H,C23-H),1.98(m,2H,C18-H),0.75(m, 3H,C19-H); Anal. Calcd ForC 29 h 24 N 4 o 8 S: C, 59.18; H, 4.11; N, 9.52. Found: C, 59.12; H, 4.02; N, 9.44. MS-ESI m / z: 611.5 [M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com