Novel crystal form D of Retigabine and preparation method thereof
A technology of retigabine and crystal form, applied in the field of medicinal chemistry, can solve the problems of uncertain bioavailability, non-specific crystal form, difficult quality control, etc., to avoid pollution and change of drug properties, reduce production costs, The effect of reducing production links
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
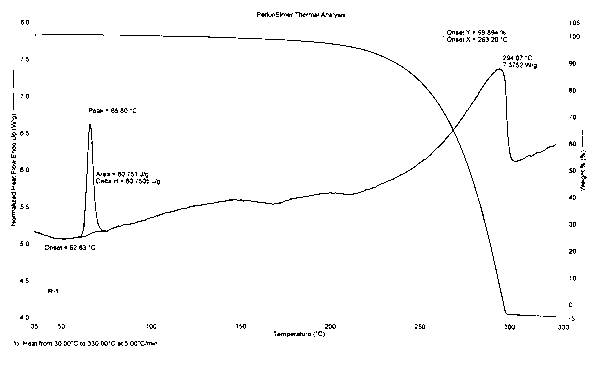
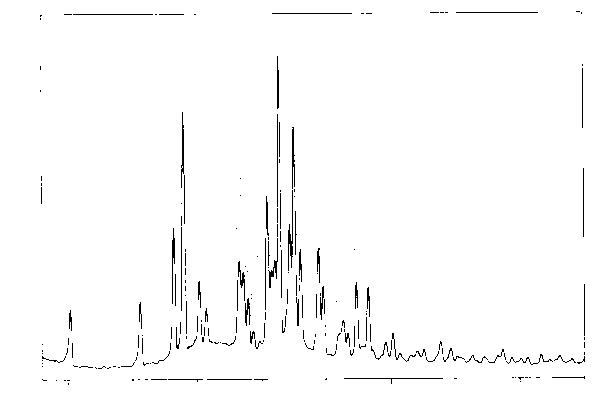
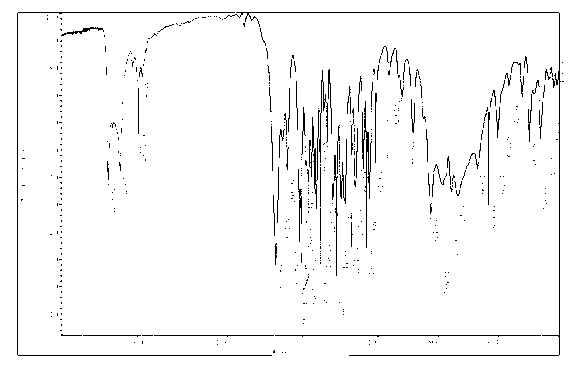
Image
Examples
Embodiment 1
[0028] Add 2kg retigabine and 8L diethyl ether into the dissolving tank, stir at room temperature until the solids are completely dissolved, filter, pump the filtrate into the crystallization tank, cool to -10°C to 0°C, stir and crystallize for 4 hours, and filter with a centrifuge. Washed with ether for 3 times, spin-dried, and dried under reduced pressure to obtain 1.83 kg of new crystal form D of retigabine.
Embodiment 2
[0030] Add 2kg retigabine and 8L diethyl ether into the dissolution tank, stir at room temperature until the solids are completely dissolved, filter, pump the filtrate into the crystallization tank, cool to 0°C to 10°C, stir and crystallize for 4 hours, filter with a centrifuge, diethyl ether Washed 3 times, dried, and dried under reduced pressure to obtain 1.85 kg of retigabine new crystal form D.
Embodiment 3
[0032] Add 2kg retigabine and 12L isopropyl ether into the dissolution tank, stir at room temperature until the solids are completely dissolved, filter, pump the filtrate into the crystallization tank, cool to -20°C to -10°C, stir and crystallize for 4 hours, centrifuge Machine filtration, washed 3 times with isopropyl ether, dried, and dried under reduced pressure to obtain 1.87kg retigabine new crystal form D.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com