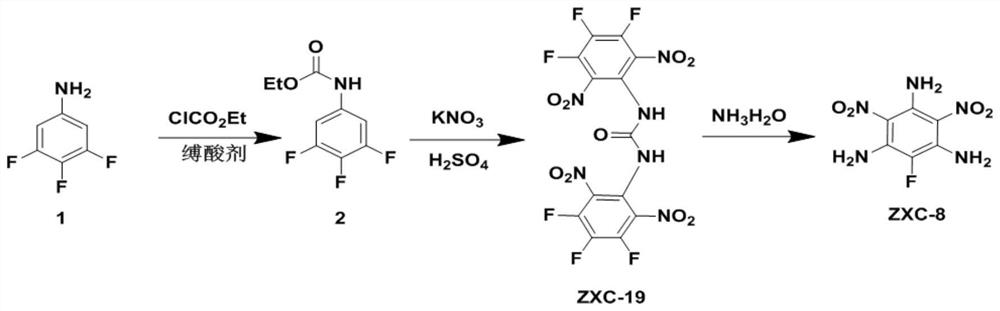
Energetic material 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl)urea and its preparation method and application
A technology of dinitrophenyl and urea, applied in the field of compound preparation, can solve the problems of inconvenient transportation, low yield and the like, and achieve the effects of simple equipment, high yield and safe raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
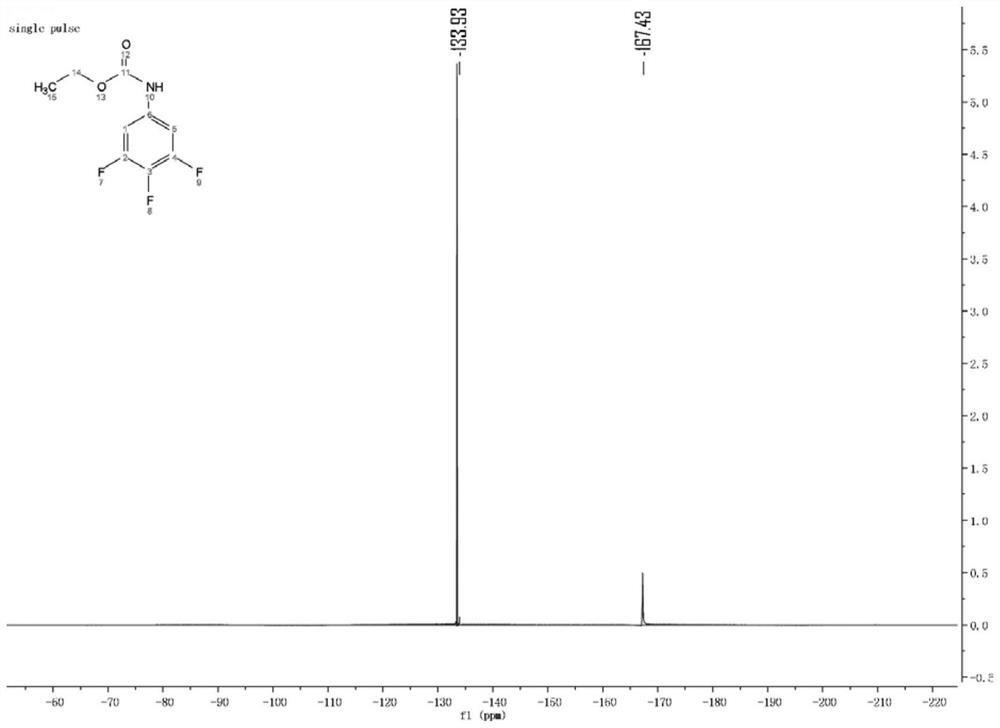
[0026] Preparation of raw material 1:
[0027] Under stirring in an ice-water bath, add 3,4,5-trifluoroaniline (0.1mol) and N,N-dimethylformamide (150mL) into a 250mL round-bottomed flask in sequence to dissolve the 3,4,5- Trifluoroaniline, then potassium carbonate (16.56g) was added, and ethyl chloroformate (0.12mol) was slowly added dropwise. After the initial exotherm was completed, the ice-water bath was removed, and the temperature was slowly raised to room temperature to react for 24 h, then the reacted mixture was poured into ice water, filtered, washed with water, and dried to obtain a gray solid (19.56 g, yield 89.31%).
Embodiment 2
[0029] A preparation of energetic material 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl)urea (ZXC-19), comprising the following steps:
[0030] Accurately weigh 5.56 grams of potassium nitrate (55mmol, 2.2eq), and slowly add it to 50 ml of concentrated sulfuric acid (the concentration of sulfuric acid is 95-98%) under ice-water bath magnetic stirring conditions, and keep the reaction for 10 minutes under ice-water bath conditions , slowly added raw material 1 (5.479g, 25mmol, 1.0eq), after the addition, the system was slowly raised to room temperature and continued to stir until the reaction was complete (TLC detection).
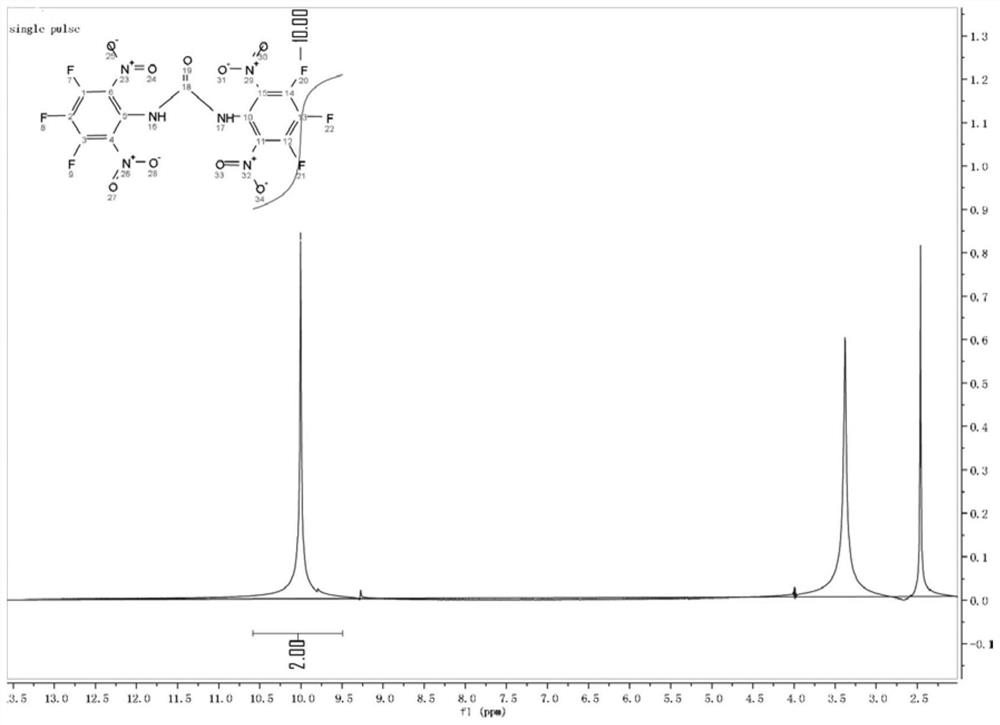
[0031] After the reaction was complete, the reaction mixture was slowly poured into vigorously stirred ice water, a large amount of precipitates were precipitated, filtered, washed with water, and dried to obtain a light yellow solid with a yield of 89%; the H NMR spectrum of the product was 1 H NMR (600MHz, CDCl 3 -d 1 )δppm: 10.0(s,2H); 13 C NMR (151MHz, C...
Embodiment 3
[0033] A preparation of energetic material 1,3-bis(3,4,5-trifluoro-2,6-dinitrophenyl)urea (ZXC-19), comprising the following steps:
[0034]Accurately take by weighing 5.56 grams of potassium nitrate (55mmol, 2.47eq), under ice-water bath magnetic stirring condition, slowly join in 50 milliliters of vitriol oil, (concentration of sulfuric acid is 95~98%), keep reaction 10 under ice-water bath condition Minutes, raw material 1 (4.89g, 22.3mmol, 1.0eq) was slowly added, after the addition, the system was slowly raised to room temperature and continued to stir until the reaction was complete (TLC detection).
[0035] After the reaction was complete, the reaction mixture was slowly poured into vigorously stirred ice water, a large amount of precipitates were precipitated, filtered, washed with water, and dried to obtain a light yellow solid with a yield of 86.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| impact sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com