Recombinant beta-amyloid peptide B cell epitope polypeptide chimeric antigen and preparation method and application thereof
A chimeric antigen and epitope polypeptide technology, applied in the fields of biopharmaceuticals and genetic engineering, can solve the problems of low immunogenicity and difficult to achieve the expected effect, and achieve the effect of mature technology, good immunogenicity and avoiding expensive costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 16A
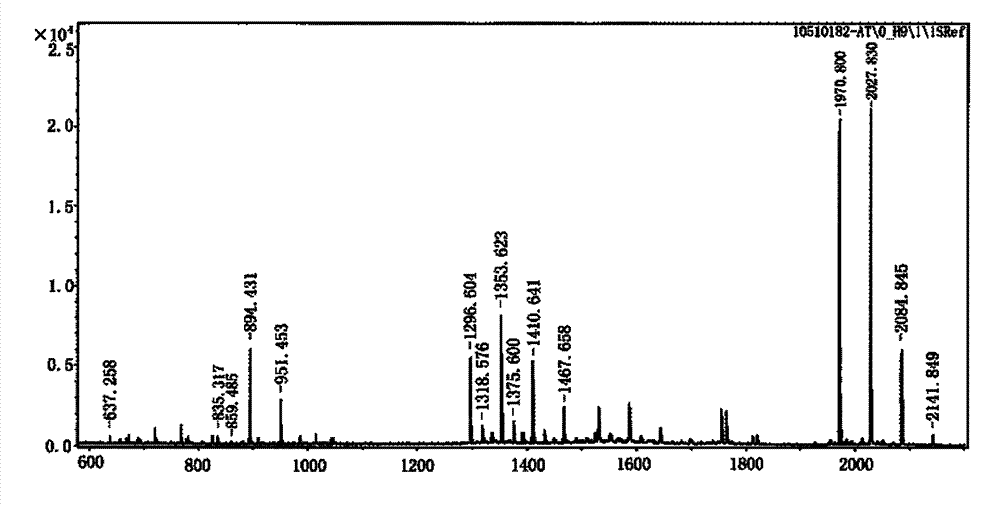
[0034] Example 16 Obtaining of Aβ15, 6Aβ15-T and THc Genes
[0035] According to the codon degeneracy, six copies of the 6Aβ15 gene (see SEQ ID No.1 in the sequence listing) were artificially synthesized to encode the AβB cell epitope polypeptide antigen (amino acids 1 to 15, DAEFRHDSGYEVHHQ, referred to as Aβ15) in tandem repetition. The direct synthetic clone was named pMD18-6Aβ15 in the pMD18-T(TaKaRa)T vector, encoding 6Aβ15 (see SEQ ID No.18 in the sequence listing for the amino acid sequence). Each Aβ15 was connected with a KL or GS flexible peptide, starting from 9 nucleotides (ATG), all the way to CAG (7th last nucleotide). Among them, DAEFRHDSGYEVHHQ is Aβ15, KL (AAGCTT) is the enzyme cutting site introduced to provide a site for the introduction of carrier molecules at the N-terminus of Aβ15 in the next step, and GS is a flexible peptide.
[0036] Then artificially synthesized the recombinant polypeptide chimeric antigen 6Aβ15-T gene (see SEQ ID No. 2), directly cl...
Embodiment 2
[0039] Example 2 Construction of prokaryotic expression vector and expression and purification of recombinant polypeptide antigen in Escherichia coli
[0040] 1. Construction of recombinant prokaryotic expression vector
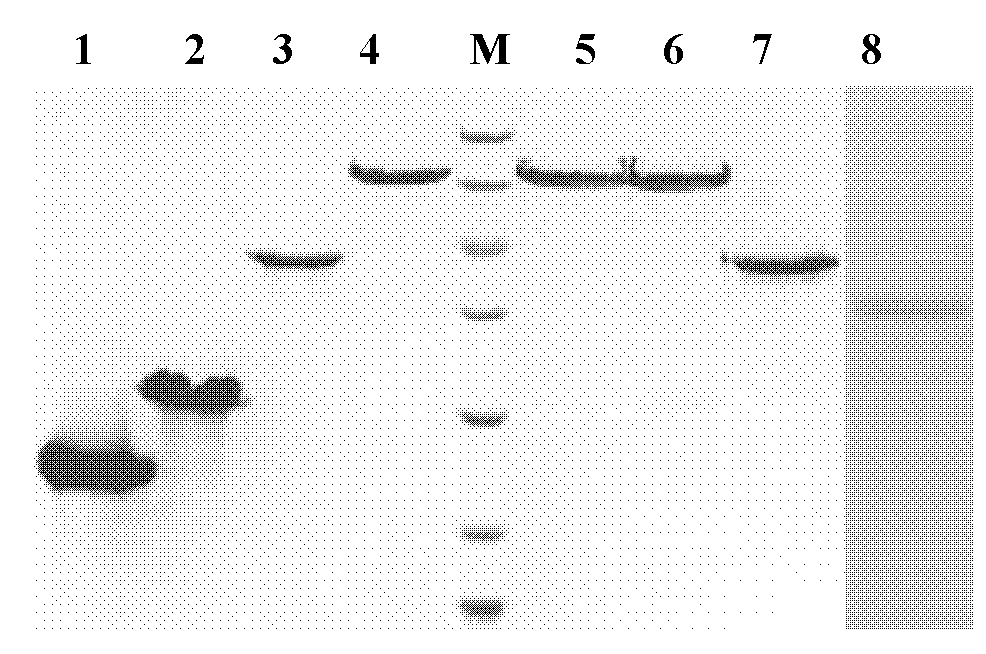
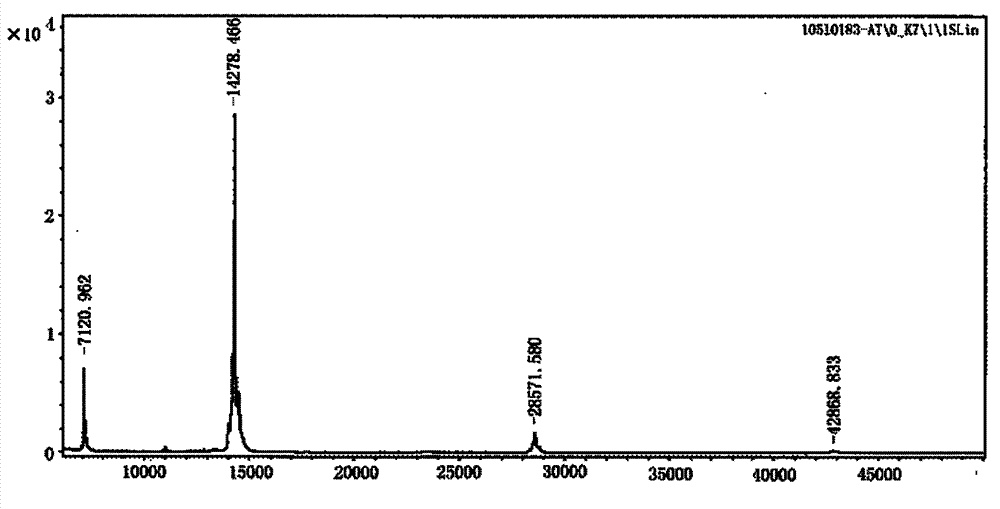
[0041] The plasmids pMD18-6Aβ15 and pMD18-6Aβ15-T obtained in Example 1 were double-digested with EcoR I and Xho I and EcoR I and Not I, respectively, and the target fragments with a length of about 320 and 350 bp were respectively recovered with a DNA recovery kit, and compared with the The prokaryotic expression vector pTIG-Trx (see patent: ZL2007100895882) was ligated with the same enzyme, and the ligated product was transformed into Escherichia coli (E.coli) DH5α competent cells, positive clones were screened, plasmids were extracted, sequenced, and the sequence and insertion position were obtained The correct recombinant prokaryotic expression vectors were named pTIG-Trx-6Aβ15 and pTIG-Trx-6Aβ15-T, respectively.
[0042]The 6Aβ15-T and the toxin fragmen...
Embodiment 3
[0055] Example 3 Recombinant Polypeptide Chimeric Antigen 6Aβ15-T Induces Common Mice to Produce High Levels of Anti-Aβ Antibody
[0056] Using the purified recombinant protein 6Aβ15 and 6Aβ15-T expressed in Example 2 as the immunogen, i.e. the subunit vaccine, to immunize mice to detect its immunogenicity, and the artificially synthesized Aβ15 dissolved in PBS (the amino acid sequence is DAEFRHDSGYEVHHQ , with a purity of more than 90%, synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.) and Aβ42 (the amino acid sequence is DAEFRHDSGYEVHHQKLVFFAED VGSNKGAIIGLMVGGVVIA, with a purity of more than 90%, synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.) polypeptide antigen for comparison. The specific method is as follows: Balb / c puppies (8 weeks old, female, SPF grade, purchased from the Experimental Animal Center of the Academy of Military Medical Sciences) were randomly divided into 5 groups, with 8 animals in each group, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com