Method of treating cancer with HDAC inhibitors
A technology of use and diluent, which is applied in the fields of pharmaceutical formulations, organic chemistry, antineoplastic drugs, etc., and can solve the problems of undisclosed favorable pharmacokinetic characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0408] Synthetic SAHA
[0409] SAHA may be synthesized as outlined below, or by the method described in US Patent No. 5,369,108, the contents of which are incorporated herein by reference in its entirety, or by any other method.
[0410] Synthetic SAHA
[0411] Step 1 - Synthesis of Suberanilic acid
[0412]
[0413] In a 22 L flask, 3,500 g (20.09 mol) of suberic acid was added, and the acid was heated to melt. The temperature was raised to 175° C., and then 2,040 g (21.92 mol) of aniline were added. The temperature was raised to 190° C. and maintained at this temperature for 20 minutes. The melt was poured into a Nalgene tank containing 4,017 g of potassium hydroxide in 50 L of water. The mixture was stirred for 20 minutes, then the melt was added. The reaction was repeated on the same scale and a second batch of the melt was poured into the same potassium hydroxide solution. After the mixture was stirred well, the stirrer was turned off and the mixture was allowe...
Embodiment 2
[0426] Oral suberoylanilide hydroxamic acid (SAHA)
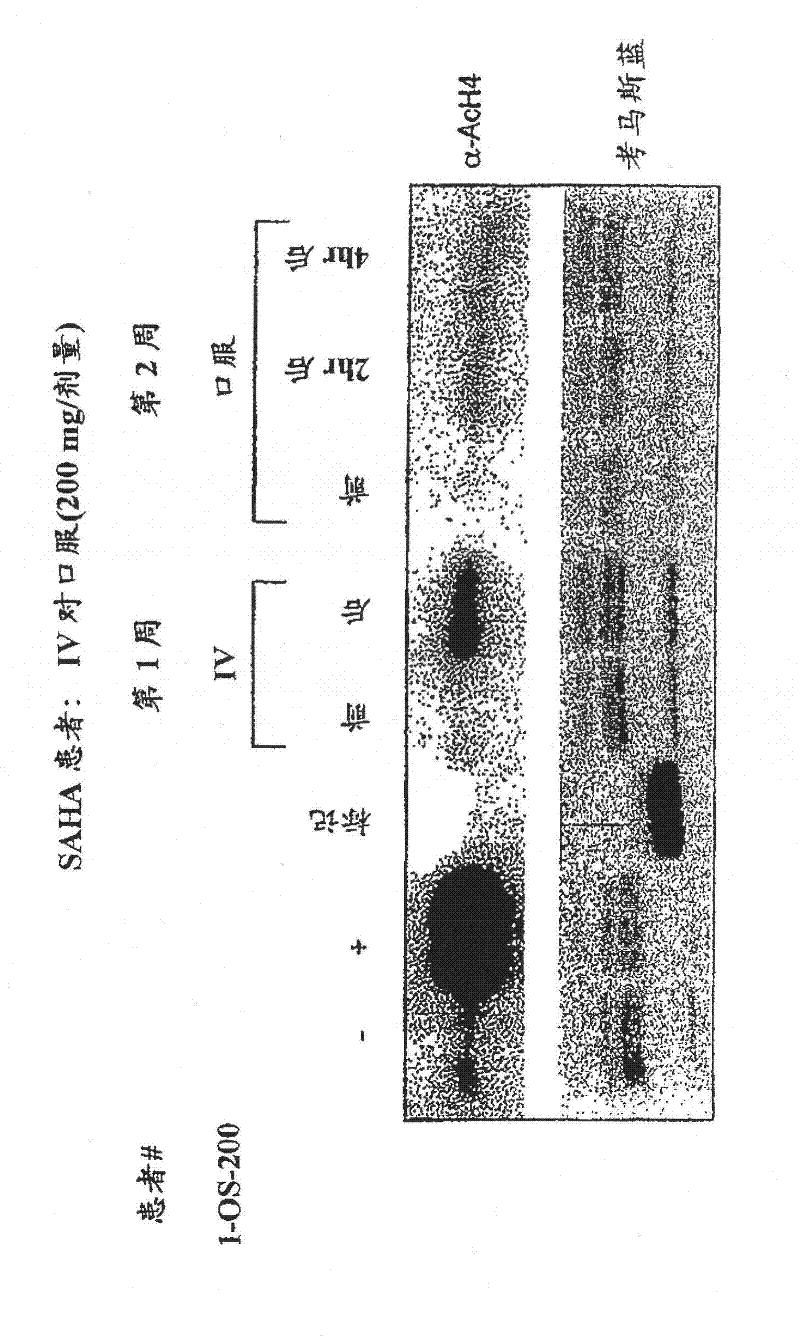
[0427] background : Treatment with hybrid polar cell differentiating agents results in growth inhibition of human solid tumor-derived cell lines and xenografts. This effect is mediated in part by inhibition of histone deacetylases. SAHA has been found in laboratory and preclinical studies to be a potent histone deacetylase inhibitor with the ability to induce tumor cell growth arrest, differentiation and apoptosis.
[0428] Purpose : To define a safe daily oral regimen of SAHA that can be used in a phase II study. In addition, the pharmacokinetic profile of the SAHA oral formulation was evaluated. The bioavailability and antineoplastic therapeutic effects of oral SAHA in humans were also monitored under fasted and non-fasted conditions. In addition, the biological effects of SAHA on normal tissues and tumor cells were evaluated, and the responses to histone acetylation levels were recorded.
[0429] patient : Patie...
Embodiment 3
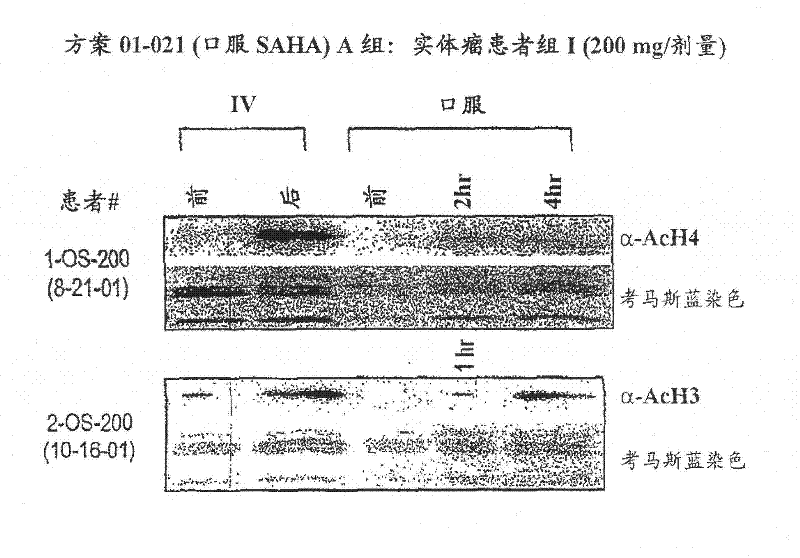
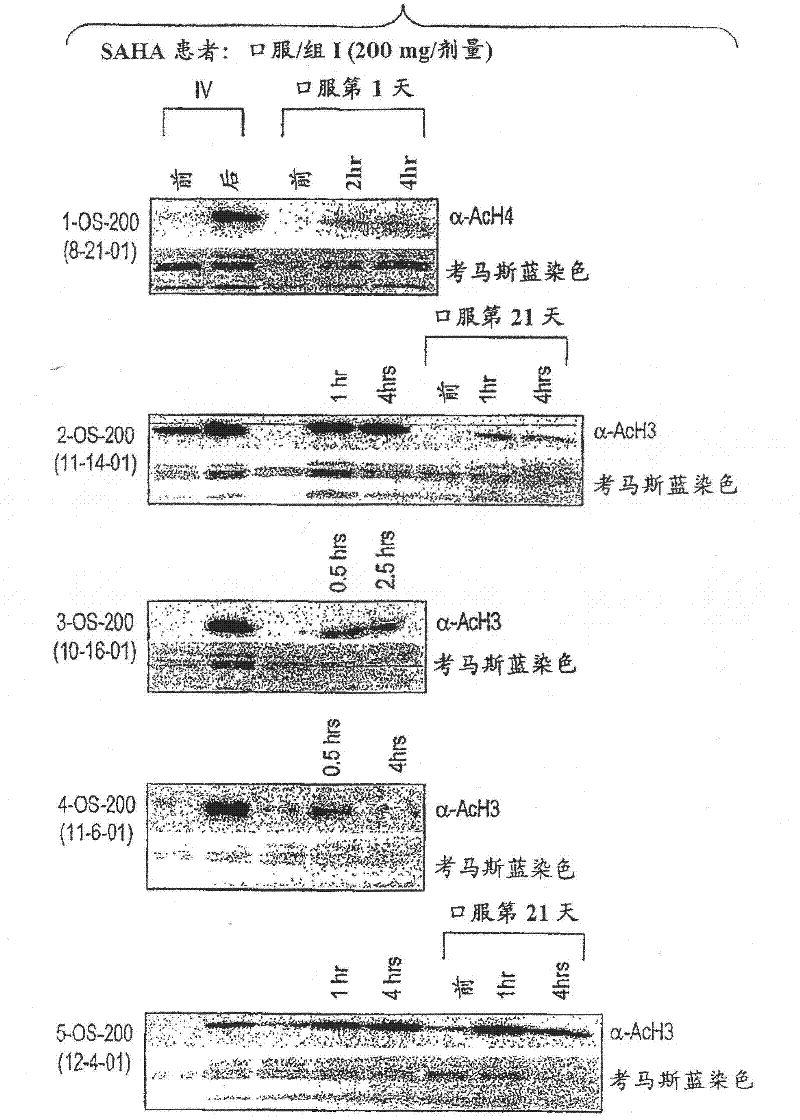
[0441] Oral suberoylanilide hydroxamic acid (SAHA) - dose escalation
[0442] In another experiment, as shown in Table 4, 25 patients with solid tumors were enrolled in group A, 13 patients with Hodgkin's or non-Hodgkin's lymphoma were enrolled in group B, and one patient with acute leukemia and one patient with myelodysplastic syndrome were enrolled in group C.
[0443] Table 4: Dose Escalation Regimen and Number of Patients by Dose Level
[0444]
[0445]
[0446] * Group A = solid tumor, Group B = lymphoma, Group C = leukemia
[0447] result :
[0448] Of the 11 patients in Group II who received treatment, 1 patient experienced Grade 3 diarrhea and Grade 3 dehydration DLT during the first treatment cycle. Nine patients were enrolled in Group III. Two patients were not eligible for 28-day toxicity evaluation and the early study was terminated due to rapid disease progression. Of the remaining 7 patients, 5 patients experienced a DLT in the first treatment cyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com