Method for determining the risk of preeclampsia using pigf-2 and pigf-3 markers
A technology of pre-eclampsia and markers, applied in the field of using PIGF-2 and PIGF-3 markers to determine the risk of pre-eclampsia, which can solve the problems of non-adoption of pre-eclampsia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
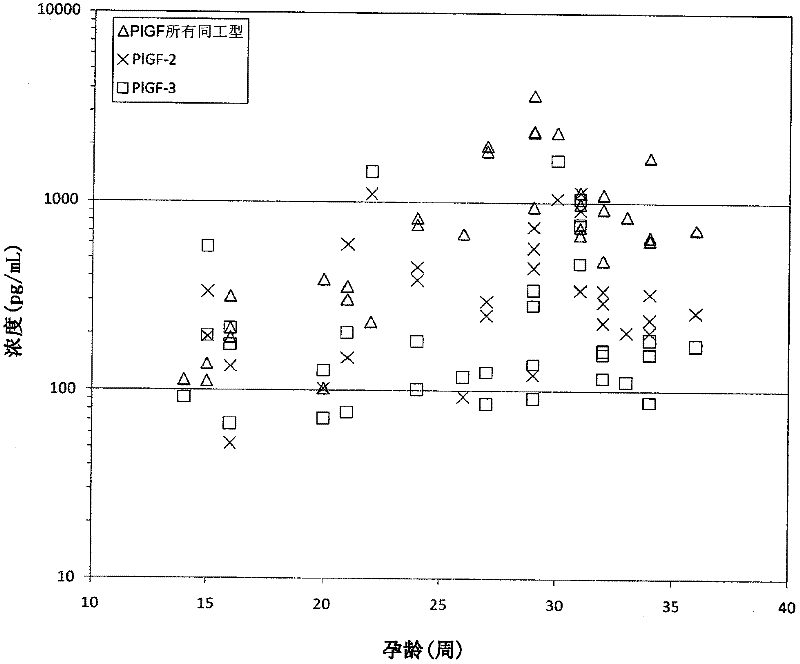
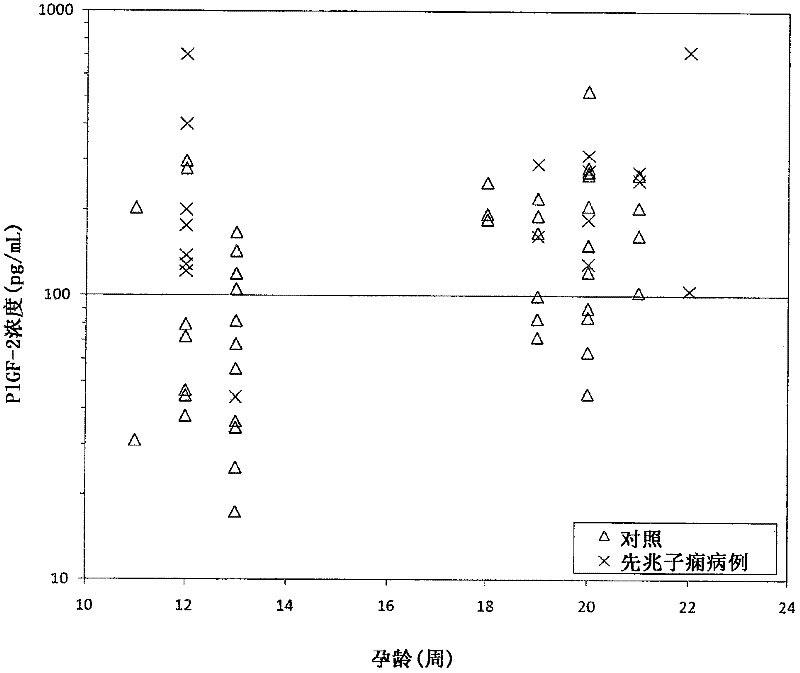
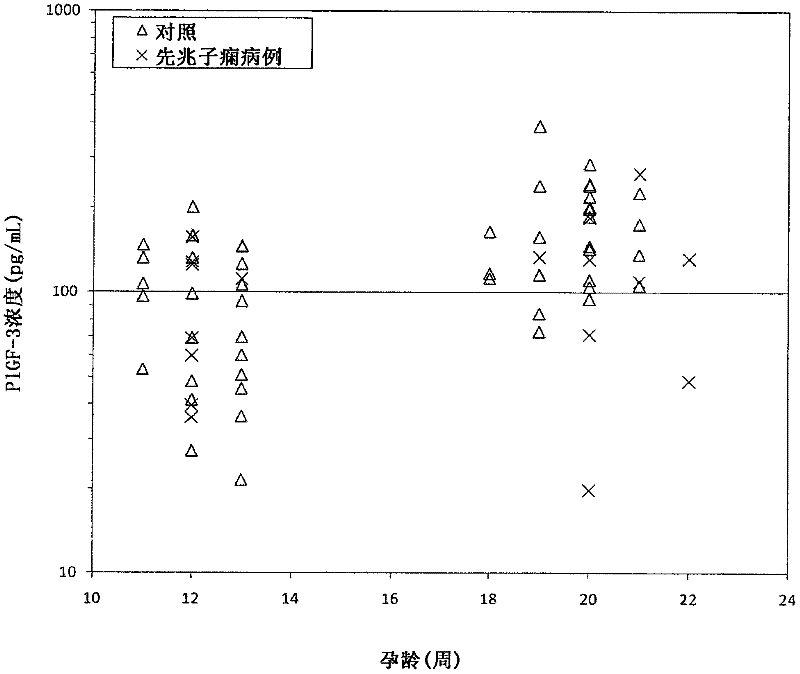
[0159] This example describes that levels of PlGF-2 in maternal serum are increased in subjects who develop preeclampsia, while levels of PlGF-3 in maternal serum are decreased in subjects who develop preeclampsia.
[0160] To measure (a) PlGF-2; (b) PlGF-3; and (c) combinations of PlGF-1, PlGF-2, and PlGF-3, a PlGF isoform-specific DELFIA sandwich assay was established.
[0161] PlGF isoforms were measured in serum obtained from pregnant women who subsequently developed preeclampsia and pregnant women who did not develop preeclampsia. Blood samples were drawn twice from each woman: once during the first trimester of pregnancy and a second time during the second trimester. Blood tubes were centrifuged and serum was collected and aliquoted. These aliquots were stored at -20°C. Selected unaffected pregnancy controls were matched to preeclamptic pregnancy cases by biophysical parameters such as maternal age, body mass index, race, and gestational age. PlGF-2 and PlGF-3 concent...
Embodiment 2
[0175] This method shows that commercially available PlGF-1 assays are cross-reactive with other PlGF isoforms.
[0176] PlGF-1 was analyzed using the commercial DELFIA Xpress PlGF method (PerkinElmer), with samples prepared to contain known amounts of purified recombinant PlGF isoforms, including recombinant PlGF-1 (non-glycosylated). As expected, the highest PlGF-1 was observed for the PlGF-1 antibody provided with the DELFIA Xpress kit (Table 2). However, significant cross-reactivity with PlGF-2 isoforms and some cross-reactivity with PlGF-3 isoforms was also observed. Therefore, this method mainly detects PlGF-1 and is not specific.
[0177] Other manufacturers have observed similar results with their current PlGF-1 approach. For example, R&D Systems reports in their method specification that their Quantikine Human PlGF ELISA Kit is 50% cross-reactive with PlGF-2 when measured against standards prepared from PlGF-1. Roche reports in their method specification that their E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com