Method for determining the effectiveness of a treatment for preeclampsia
A pre-eclampsia, effective technology, applied in the direction of material inspection products, measuring devices, instruments, etc., can solve the problems of difficult resuscitation, neuromuscular block, mother and fetus complications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: Evaluation of the effectiveness of treatment by low-dose aspirin
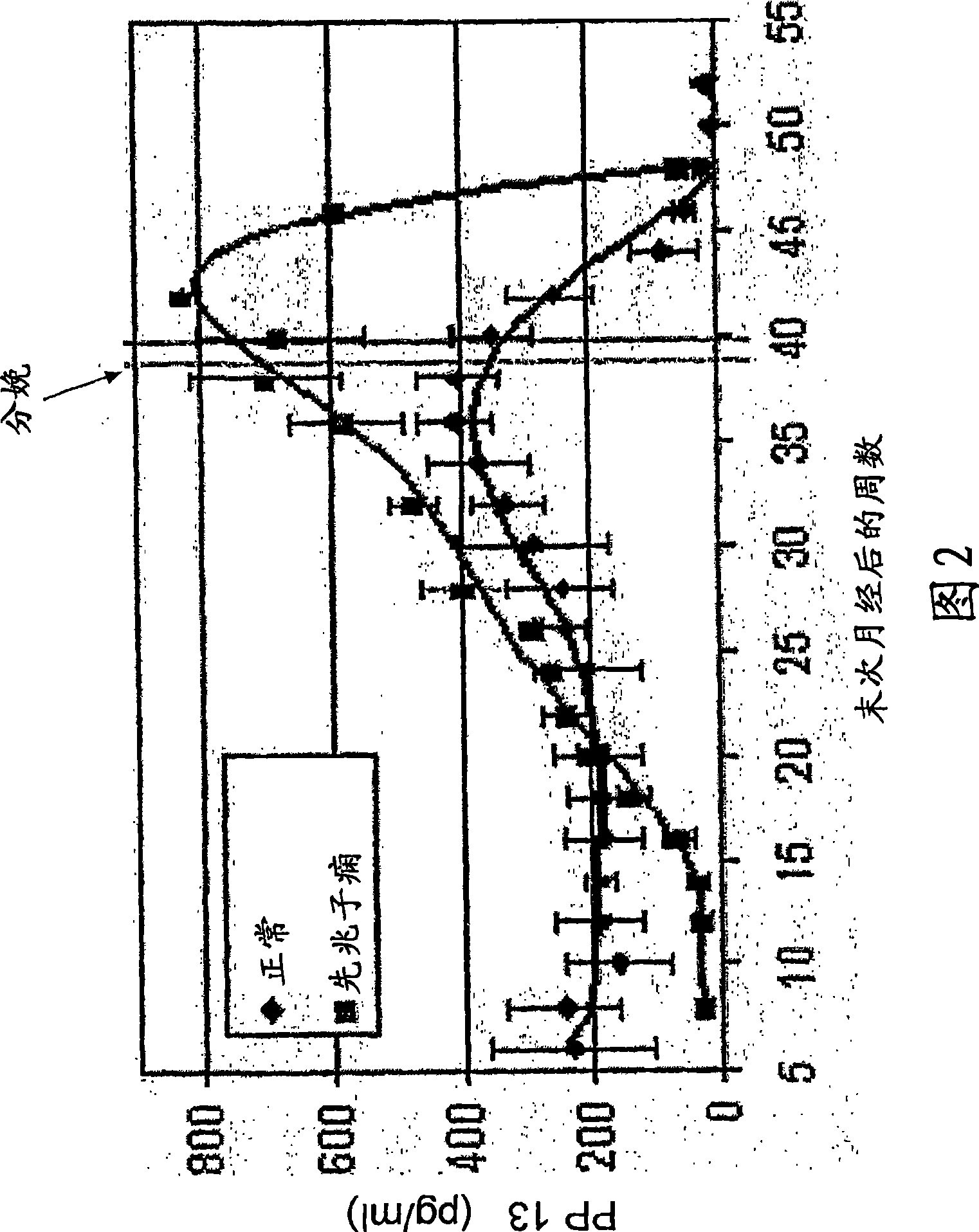
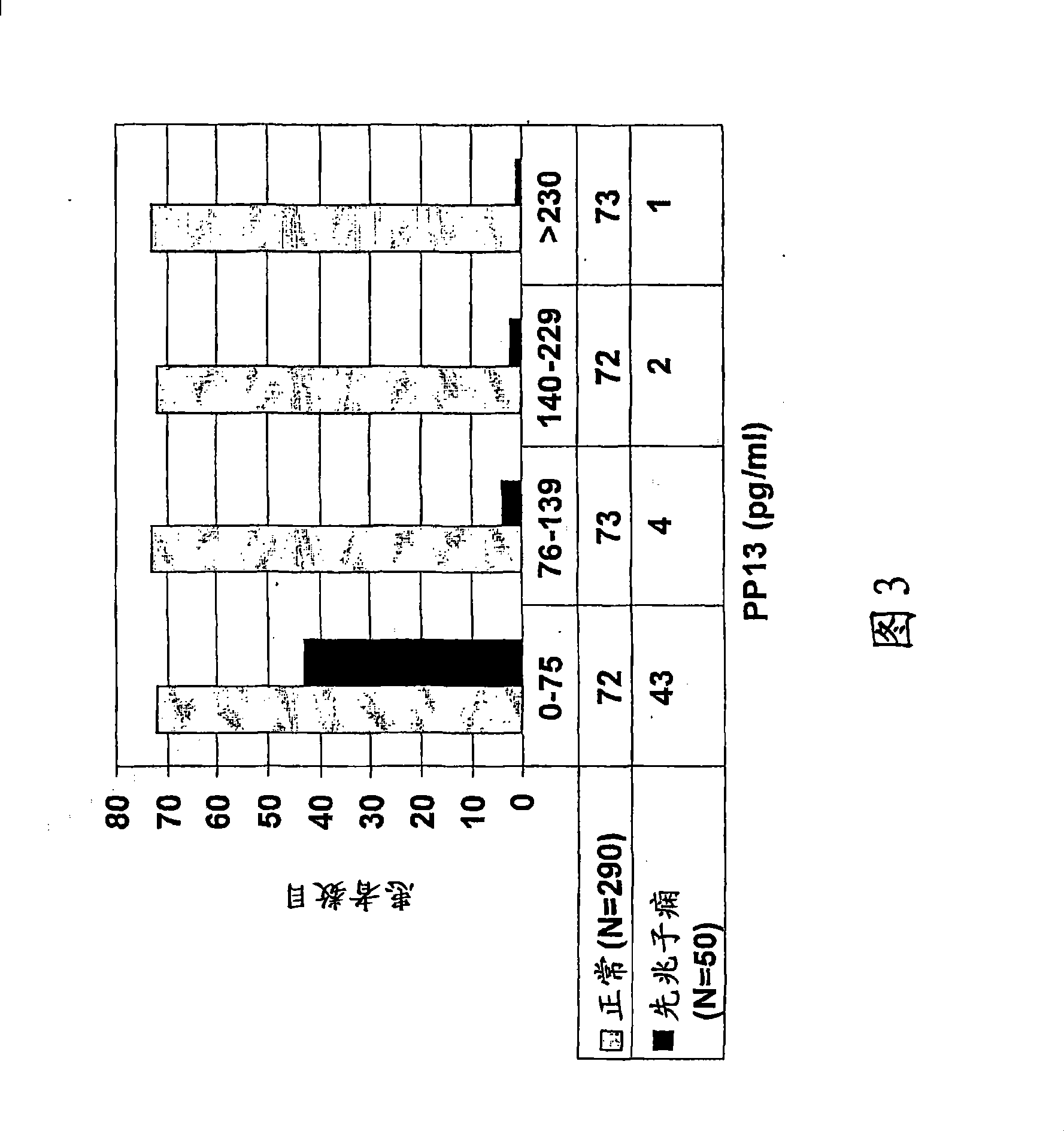
[0107] If drug therapy is applied for the periods specified above, it is expected that a woman's PP13 levels will return to the 2nd and 3rd and even 4th quartiles, corresponding to her reduced likelihood of developing preeclampsia. In the example described in Table 2, women at increased risk of developing preeclampsia were treated with aspirin 100 mg / kg (low dose aspirin) orally for 2 or 3 weeks starting from the 8th week of pregnancy. It suggests that giving aspirin early enough reduces the risk of subsequent development of preeclampsia. Therefore, women receiving treatment can expect to reduce their risk of developing preeclampsia and improve their outcomes.
[0108] As shown in Table 2, in the trial, 150 women were tested as being at high risk at week 8, 50 were untreated, 50 were treated with aspirin for two weeks and 50 were treated with aspirin for three weeks. The results showed that ...
Embodiment 2
[0113] Example 2: Evaluation of the effectiveness of treatment by anticoagulant drugs
[0114] Women considered to be at increased risk were given daily anticoagulant therapy (low molecular weight heparin, aprotinin, or other) from the eighth week of pregnancy for 2 weeks. It was found that their PP13 MoM increased to 0.48 (gestational weeks 11-15) (P<0.05) and 0.73 (gestational weeks 16-20), respectively, and the latter was practically indistinguishable from normal levels (1±0.29, median normal MoM± 95% confidence interval). PP13 MoM was not affected in normal risk women. The corresponding outcomes for treated women were: Over the entire cycle, all 5 women at increased risk developed severe preeclampsia in the no treatment group, whereas only one developed severe preeclampsia and one mild preeclampsia in the treatment group and one unaffected.
Embodiment 3
[0115] Example 3: Evaluation of Drug Benefits Using Placental Extracts
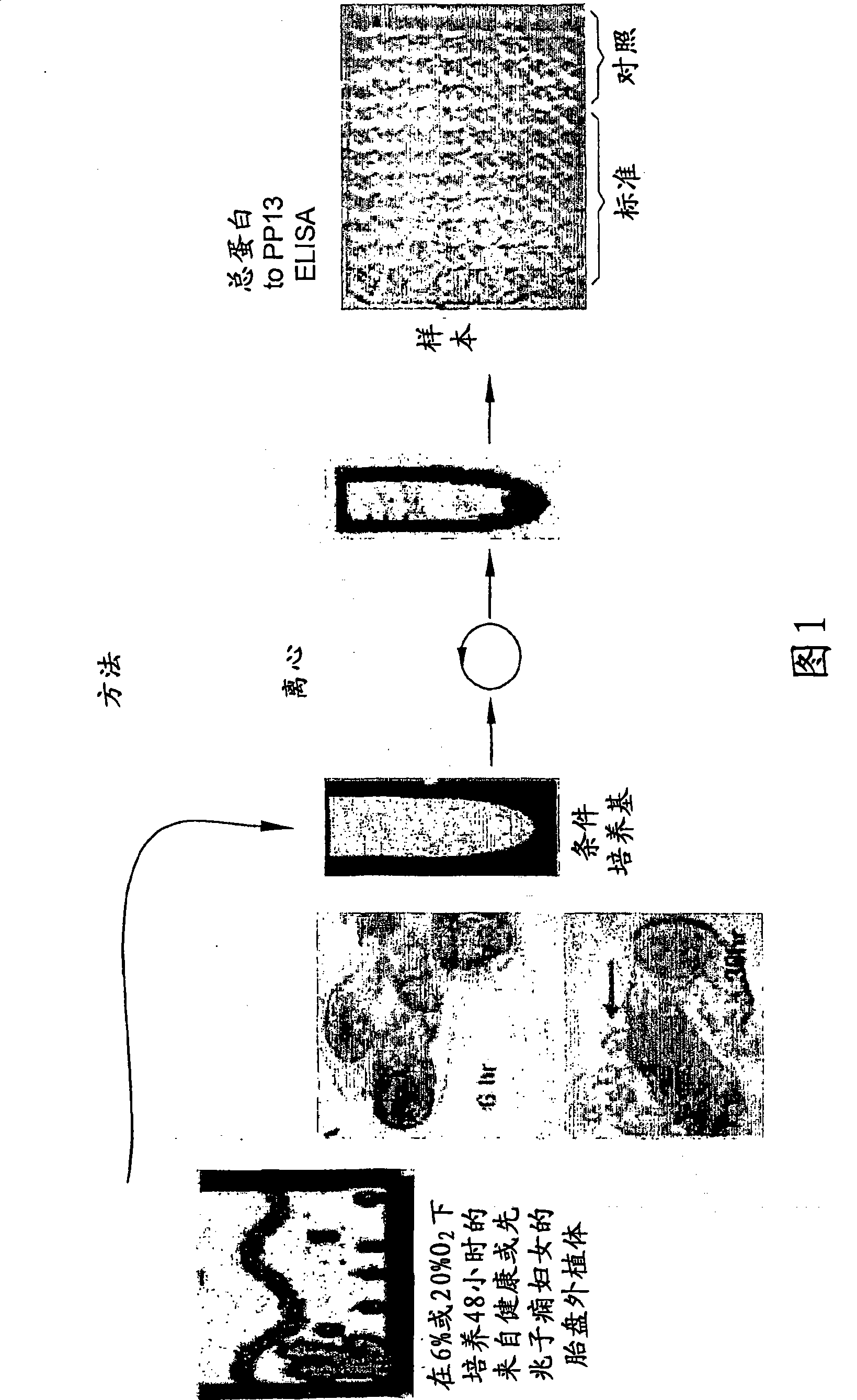
[0116] An alternate method of assessing drug benefit is through the use of placental villi (cells or explants) obtained from women at 9-10 weeks of gestation by chorionic villus sampling. Placental cells / explants were cultured for 48 hours and PP13 was assayed in the culture medium by ELISA (in the same manner as described in Figure 1). The results are shown in Table 3 below.
[0117] Table 3 shows 3 cases of preeclampsia (cases #3, 4, 5) in 6% oxygen (normoxic) compared to 14,100 and 15,700 in normal women (cases #1 and 2) In comparison, the amount released was significantly lower (3,010, 3,500 and 6,300). After 48 hours of incubation with the antioxidant vitamin C (which has shown promise in treating high-risk women), PP13 release levels returned to almost normal levels in all three high-risk women, reaching 12,030, 9,230 and 15,790 (ie 3-4 times higher). At 20% oxygen (hyperoxia), PP13 release incr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com