Eukaryotic fused expression product of two marine animal antibacterial peptide genes, and preparation method thereof
A marine animal, fusion expression technology, applied in eukaryotic fusion expression products and preparation, in the field of antibacterial peptides of Scylla pseudocaveus, can solve the problems of affecting the antibacterial activity of Hepcidin, affecting the antibacterial function of the combined polypeptide Scy-hepc, and achieve potential application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
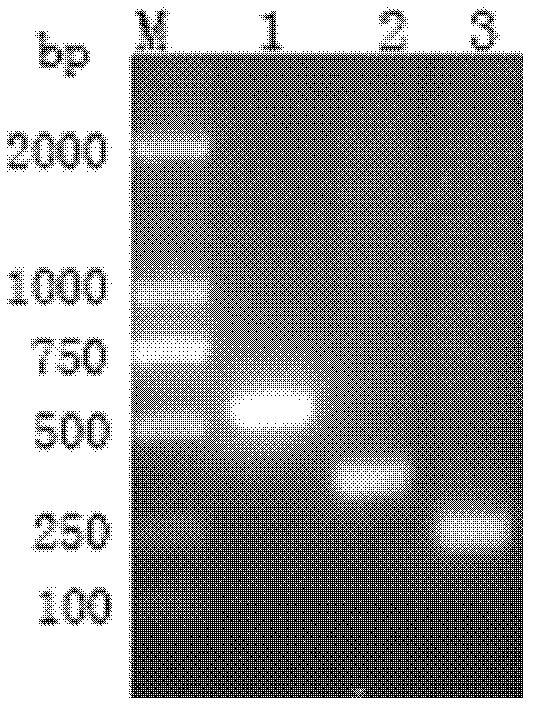
[0067] Example 1 Construction of Pichia pastoris recombinant expression plasmid pPIC9K-scy / hepc
[0068] 1) Acquisition of the scygonadin gene in the mud crab and the hepcidin gene in the large yellow croaker
[0069] According to the multiple cloning site on the pPIC9K vector, according to the cDNA sequence of Scygonadin (Genbank accession number: AY864802 ) and the cDNA sequence of large yellow croaker hepcidin (Genbank accession number: EF156401 ) Design the specific upstream and downstream primers of scygonadin and hepcdin combined polypeptide. The connecting peptide (linker) of the two tandem expression genes is 6 amino acid sequences: GGPGSG, and the corresponding codon sequence is (according to the codon preference of Pichia pastoris [4,5] Codon optimized): 5'GGTGGCCCAGGTTCCGGT3'.
[0070] F1: 5′GGG GGCCAGGCACTCAACAA3', black italics indicate the introduced EcoR I restriction site.
[0071] A connecting peptide gene sequence containing 6 amino acids was added to...
Embodiment 2
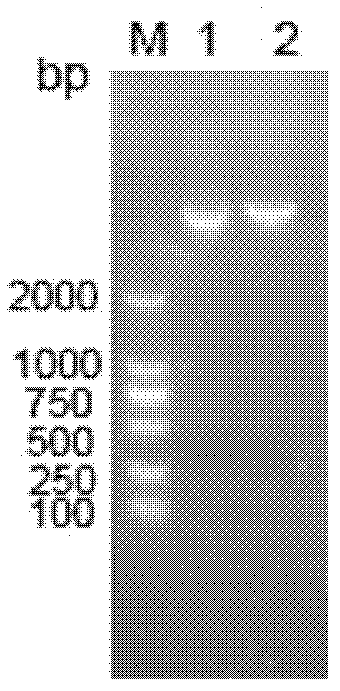
[0099] Example 2 Induced expression of pPIC9K-scy / hepc recombinant plasmid in Pichia pastoris GS115
[0100] 1) Linearization of pPIC9K-scy / hepc
[0101] The strains containing the expression vector with correct sequencing were streaked and cultured, and the single clones were picked and shaken for culture. After the plasmid was extracted, 10-20 μg of the plasmid was linearized by the Sac I restriction endonuclease. The reaction system was as follows:
[0102]
[0103] The linearized pPIC9K-scy / hepc was recovered with a nucleic acid co-precipitation agent. Then it was transformed into Pichia pastoris GS115 competent cells by electric shock method, and its expression was induced.
[0104] 2) Induced expression of combined polypeptide Scy-hepc
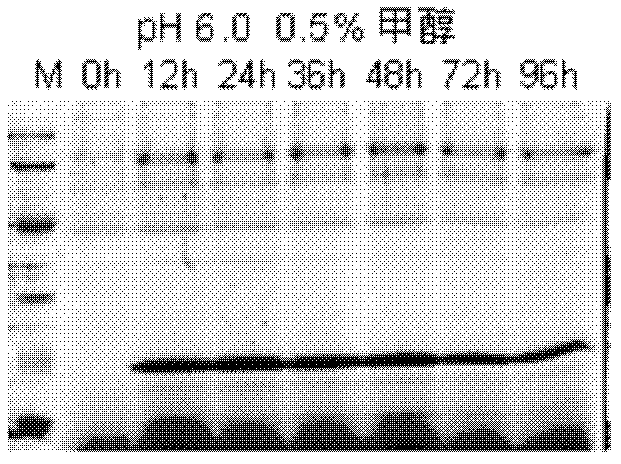
[0105] (1) Induced expression of Scy-hepc (changes in expression at different induction times):
[0106] ①Select several positive clones grown on MD plates and inoculate them in 6ml of BMGY (pH6.0) medium, culture at 29°C and 230rp...
Embodiment 3
[0114] Example 3 Purification of target protein Scy-hepc by affinity chromatography
[0115] 1) Preparation of samples before column loading
[0116] After pPIC9K-scy / hepc recombinant plasmid transformed Pichia pastoris GS115, under the condition of pH 6.0, 0.75% methanol induced expression for 36 hours, centrifuged to remove the precipitate, and collected the fermentation supernatant. At 4°C, dialyze the Pichia pastoris induced expression supernatant with PBS dialysate (50mM phosphate buffer + 50mMNaCl, pH9.0) for 2 to 3 times, centrifuge, collect the supernatant, filter through a 0.45μm membrane filter, and put it on the column for purification .
[0117] 2) Purify the target protein Scy-hepc by affinity chromatography
[0118] Affinity purification with nickel ion chelate affinity chromatography column, chromatographic reagents are:
[0119] Solution A: 20mM phosphate buffer + 50mM NaCl + 10mM imidazole, pH8.5;
[0120] Solution B: 20mM phosphate buffer + 500mM NaCl + 1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap