Fusion protein used for inhibiting regeneration or growth of blood vessel and medical treatment application thereof
A fusion protein, immunoglobulin technology, applied in cardiovascular system diseases, peptide/protein components, hybrid peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1. Cloning of DNA sequences encoding optimized fusion proteins and construction of recombinant vectors
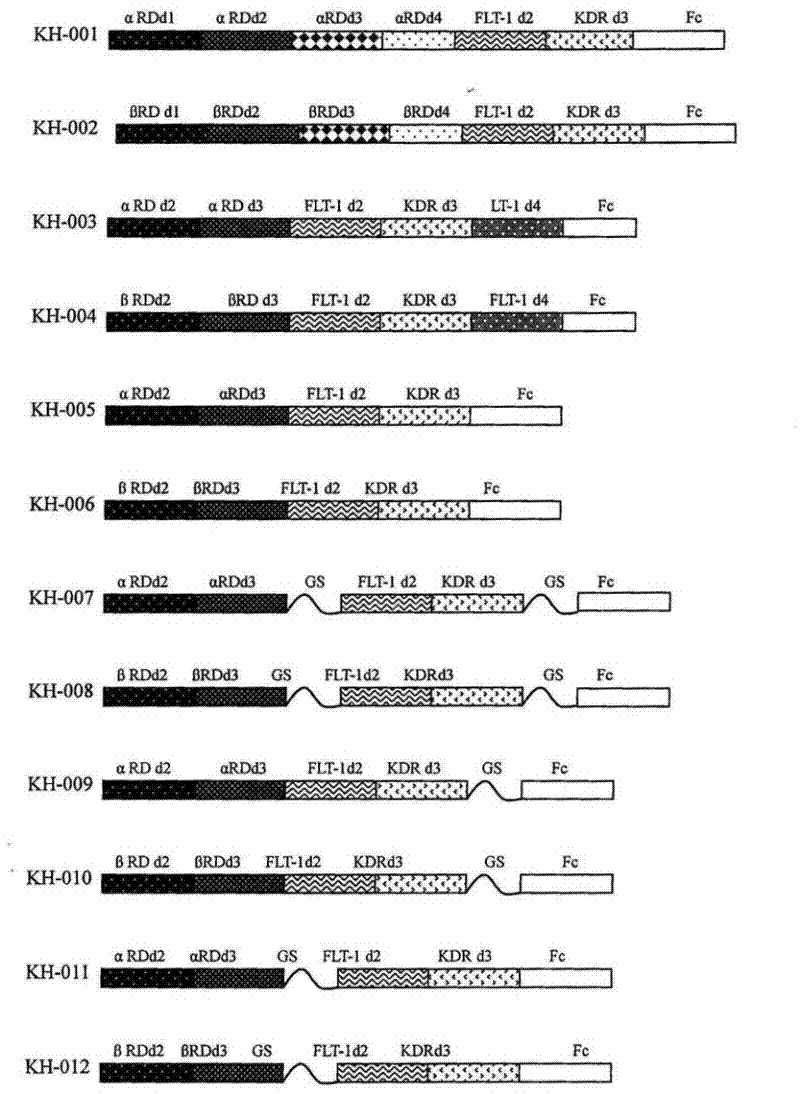
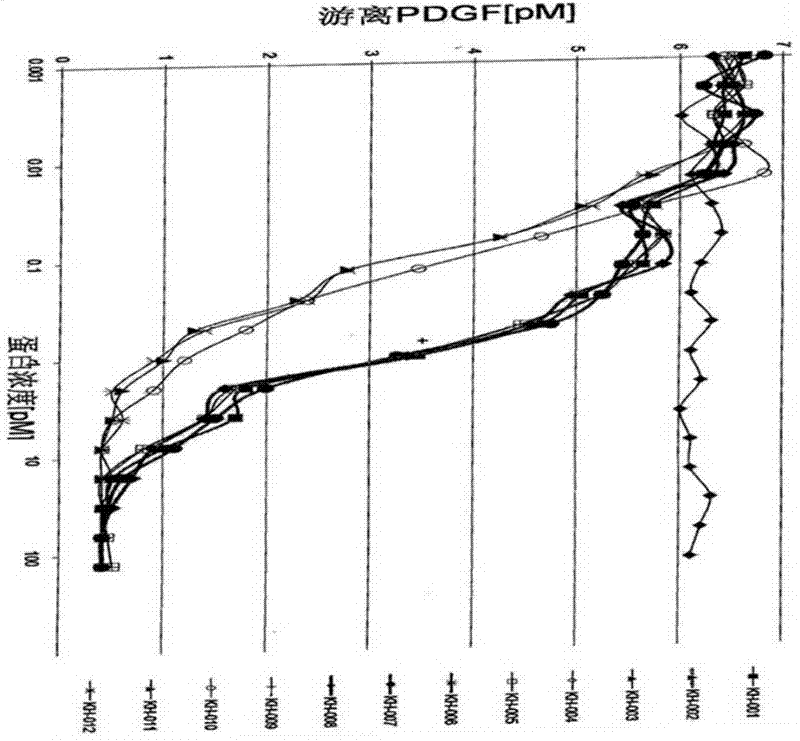
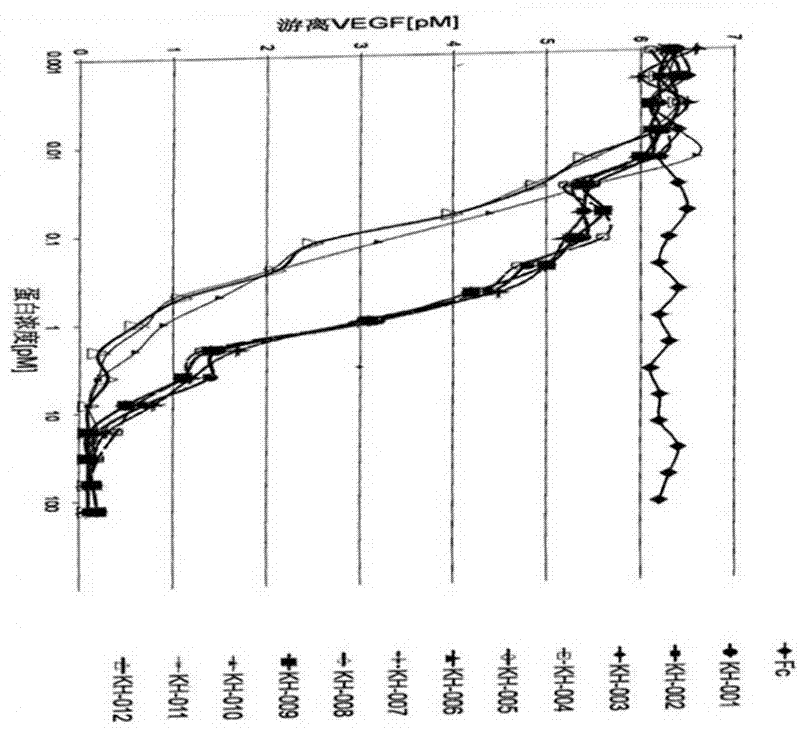
[0080] The FP2 and FP3 (ZL200510073595.4) constructed by our company were used to amplify FLT-1D2, KDRD3, FLT-1D4 and Fc respectively. The immunoglobulin-like domains encoding PDGFRα and PDGFRβ were obtained from human embryonic liver cells, and then used Different primers use polymerase chain reaction (PCR) to amplify the required fragments, and finally fuse the corresponding sequences to construct the DNA sequences of different fusion proteins. The structures of the 12 fusion proteins constructed in this preferred embodiment are shown in the appendix figure 1 .
[0081] Collect the well-growth extracted human embryonic liver cells (from the American standard cell bank) in the T-175 culture flask, about 1×10 7 For each cell, the total RNA of the cells was extracted using an RNA extraction kit (QIAGEN), and reverse-transcribed into cDNA using an Invitrogen...
Embodiment 2
[0133] Embodiment 2, the stable expression of fusion protein in CHO cell
[0134] The recombinant high-purity plasmids encoding KH-001 to KH-008 were introduced into CHO cells (Invitrogen) by electroporation, and G148 was added after two days in the serum-free culture medium. After the formation of G148 antibody colonies, the limited dilution method was used For clonal culture, after about 2 weeks, monoclonal cells were picked and transferred to culture dishes for expansion.
Embodiment 3
[0135] Purification of embodiment 3 fusion protein
[0136] The cell culture solution containing the fusion protein is collected, the cell fragments are taken out by centrifugation, and purified by staphylococcus protein A affinity chromatography. After protein A-Sepharose chromatography column was washed with PBS buffer to balance, the ultrafilter concentrated culture solution was loaded at a speed of 2 ml / min, and washed with PBS buffer until all unbound proteins were eluted (monitored by A280), then elute the bound protein with 100mM citric acid (pH=3), and immediately neutralize it with enough 2M Tris.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com