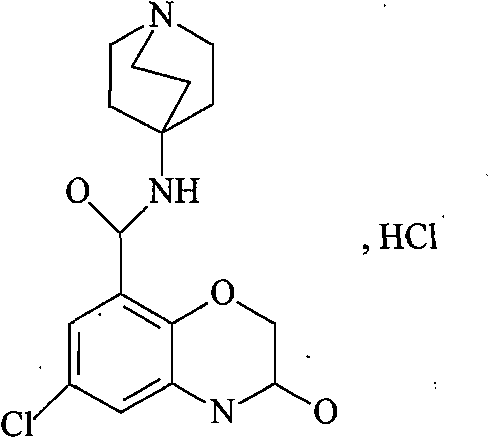
Azasetron hydrochloride lipidosome injection
A technology of injection and agar, which is applied in the field of medicine, can solve the problems of low bioavailability, low stability, and easy decomposition without overcoming, and achieve the effects of simple preparation method, increased retention time, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Azasetron Hydrochloride Liposomal Injection
[0033] Prescription (1000 bottles):
[0034]
[0035] Preparation Process:
[0036] (1) Take 10g azasetron hydrochloride, 100g cephalin, 50g cholesterol, 20g Span 80 and 5g sodium thiosulfate by weight components and place them in a pear-shaped bottle, add in 500ml acetone, heat and stir to disperse evenly, Acetone was removed under reduced pressure on a rotary evaporator to obtain a phospholipid film;
[0037] (2) Take by weighing 80g mannitol and 80g dextran and add 500ml water to dissolve, then pour into a pear-shaped bottle and shake gently, so that the phospholipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0038] (3) Pour the above-mentioned suspension into the homogenizer, and continue to homogenize;
[0039] (4) Remove the bacteria and heat source through the ultrafiltration membrane of the above-mentioned homogenized suspension, then circulat...
Embodiment 2
[0040] Example 2 Azasetron Hydrochloride Liposomal Injection
[0041] Prescription (1000 bottles):
[0042]
[0043]
[0044] Preparation Process:
[0045] (1) Take 10g azasetron hydrochloride, 150g cephalin, 100g cholesterol, 10g Span 80 and 2g sodium thiosulfate by weight components and place them in a pear-shaped bottle, add in 800ml acetone, heat and stir to disperse evenly, Acetone was removed under reduced pressure on a rotary evaporator to obtain a phospholipid film;
[0046] (2) Take 100g mannitol and 100g dextran by weight and add 500ml water to dissolve, then pour into a pear-shaped bottle and shake gently, so that the phospholipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0047] (3) Pour the above-mentioned suspension into the homogenizer, and continue to homogenize;
[0048] (4) Remove the bacteria and heat source through the ultrafiltration membrane of the above-mentioned homogenized suspension, ...
Embodiment 3
[0049] Example 3 Azasetron Hydrochloride Liposomal Injection
[0050] Prescription (1000 bottles):
[0051]
[0052] Preparation Process:
[0053] (1) Weigh 10g of azasetron hydrochloride, 50g of cephalin, 80g of cholesterol, 50g of Span 80 and 8g of sodium thiosulfate in a pear-shaped bottle by weight, add 500ml of acetone, heat and stir to disperse evenly , Remove acetone under reduced pressure on a rotary evaporator to prepare a phospholipid film;
[0054] (2) Take by weight 90g of mannitol and 90g of dextran and add 500ml of water to dissolve it and pour it into a pear-shaped bottle and shake gently, so that the phospholipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0055] (3) Pour the above-mentioned suspension into the homogenizer, and continue to homogenize;
[0056] (4) Remove the bacteria and heat source through the ultrafiltration membrane of the above-mentioned homogenized suspension, then circulate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com