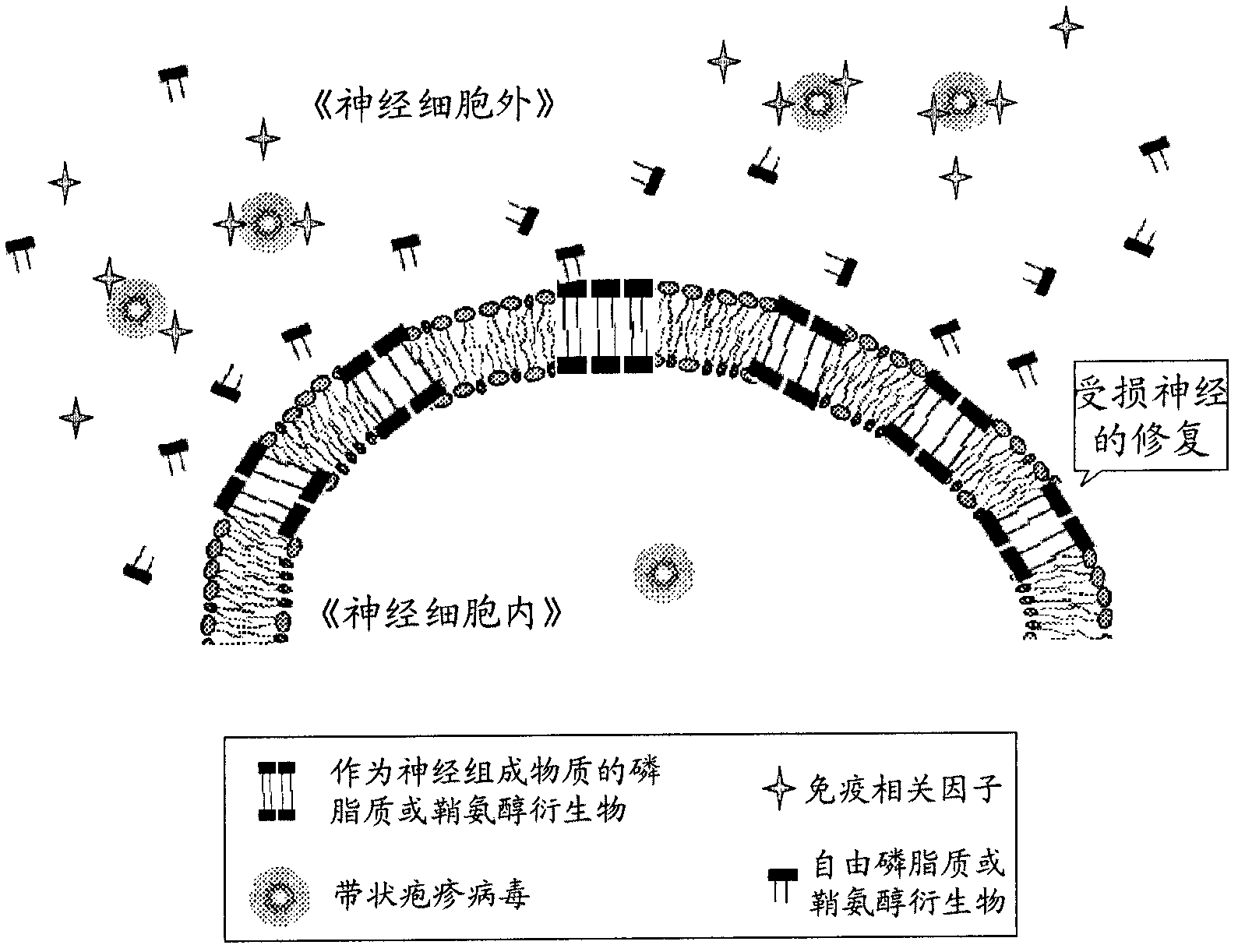
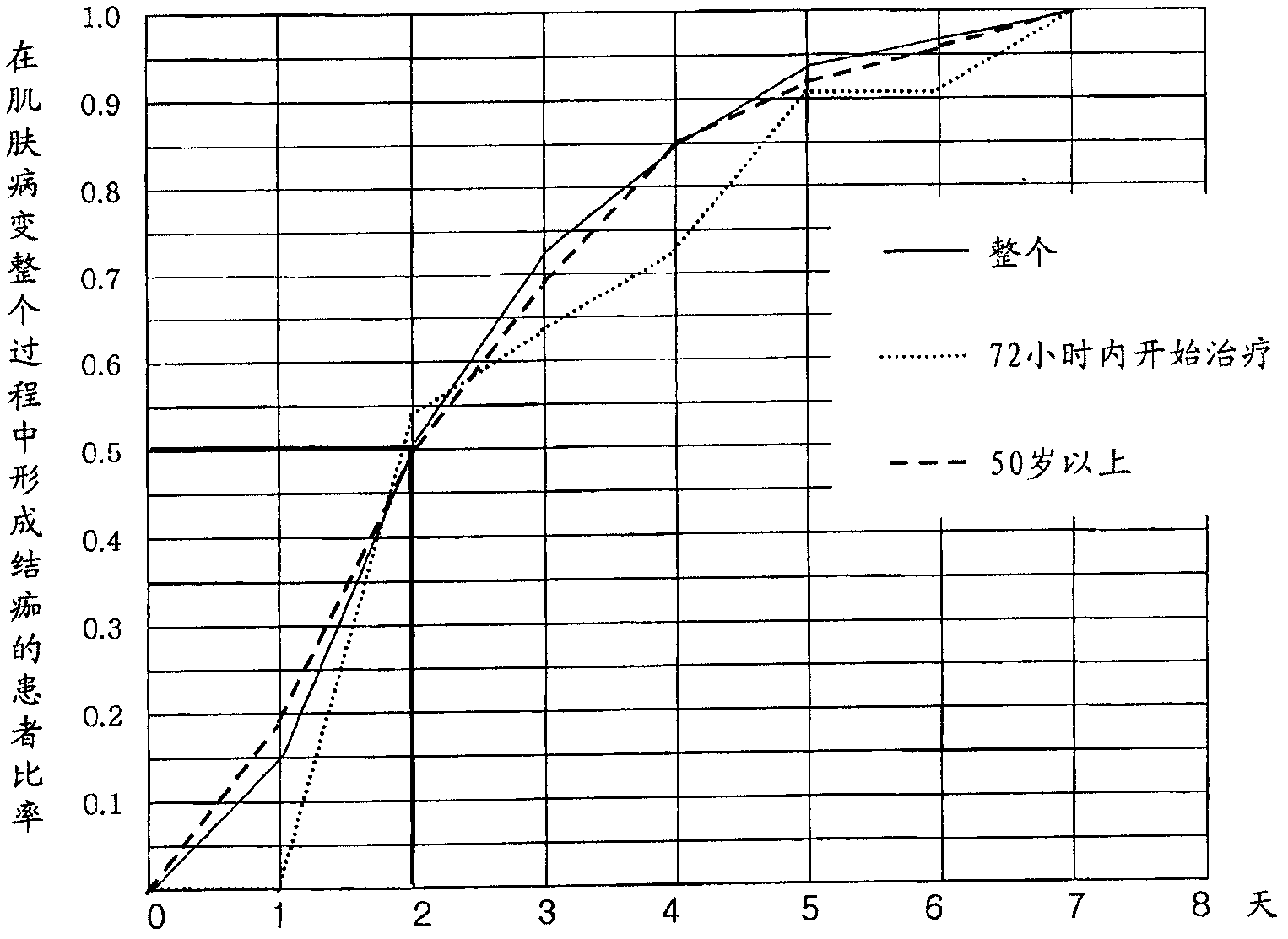
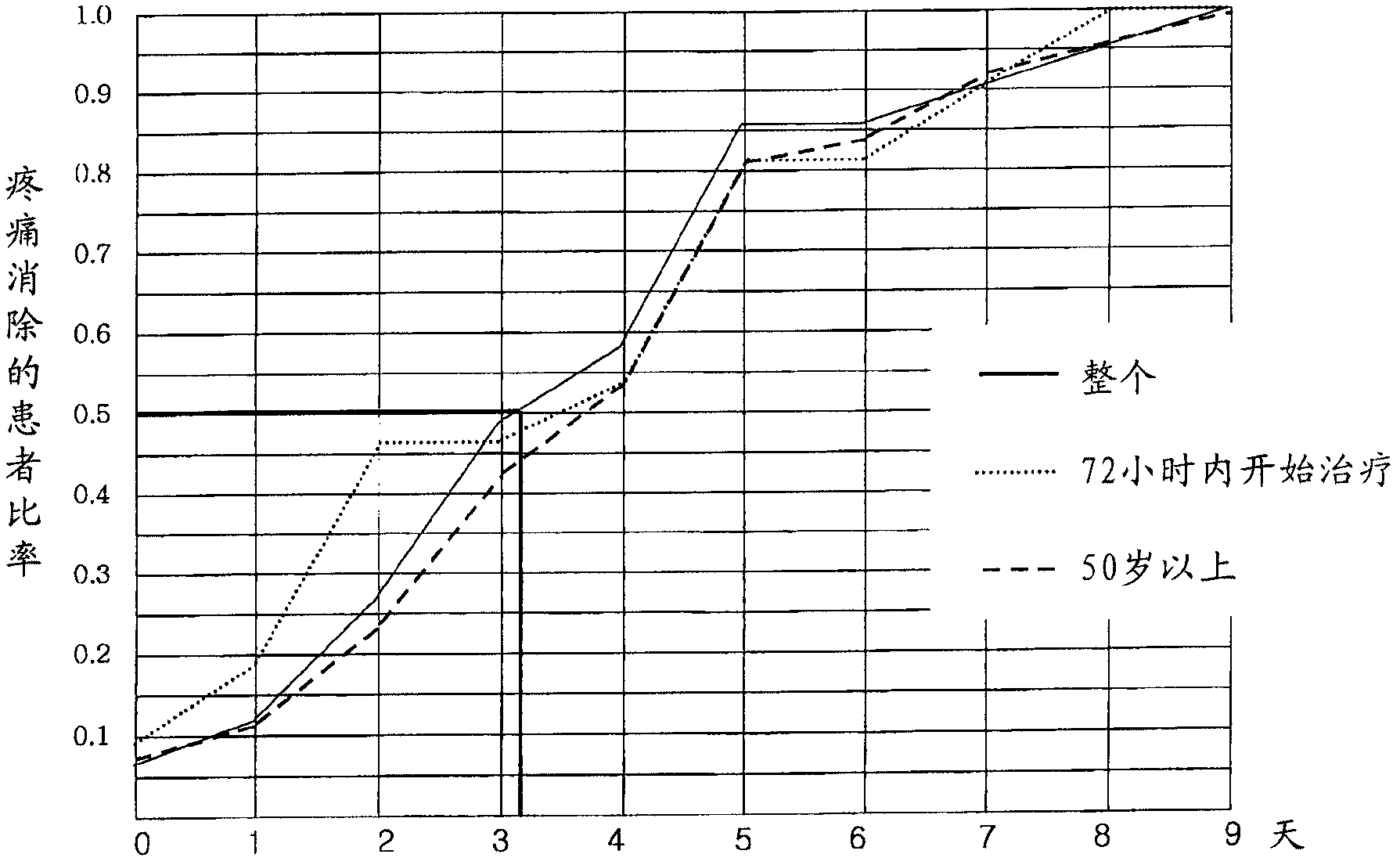
Pharmaceutical composition for preventing or treating neuronal damage and neurological diseases
A technology for nervous system diseases and nerve damage, which can be used in nervous system diseases, cardiovascular system diseases, drug combinations, etc., and can solve problems such as the inability to effectively regulate pain, and the inability to repair nerve tissue damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of H-Z Cream
[0063] Put 15g of stearic acid into a 250mL beaker, melt it completely in a water bath at 80-85°C, add 75mL of purified water to another 200mL beaker, dissolve 0.7g of potassium hydroxide and 10mL of glycerin, and heat it to the solution at 80-85°C, stirred, and then cooled to normal temperature to prepare vanishing cream.
[0064] In 229.0 g of aloe vera gel, add 100.7 g of the above-prepared cream, stir well, and then slowly add 16.03 g of ORONIA soybean lecithin (provided by Terrako Natural Source Company Ltd.) as a phospholipid or a sphingosine derivative , 1.60g α-tocopherol and 0.50g propyl benzoate (propyl benzoate), while stirring, prepare beige cream. At this time, the prepared composition contained 1.7% by weight of phospholipids or sphingosine derivatives.
Embodiment 2
[0065] Preparation of H-Z Cream
[0066] In 200.0g aloe vera gel, add 4.00g polysorbate #80, stir well, then slowly add 20.00g ORONIA soybean lecithin (supplied company: Terrako Natural Source Company Ltd.), 2.00g α-tocopherol and 0.30 g of propyl benzoate was stirred to prepare a beige cream. At this time, the prepared composition contained 3.24% by weight of phospholipids or sphingosine derivatives.
Embodiment 3
[0067] Preparation of H-Z Cream
[0068] Add 14.00 g of polysorbate #80 to 127.0 g of aloe vera gel, stir well, and then slowly add 70.00 g of GL-90E (provided by: Goshenbiotech Inc.), 7.00 g of phospholipids or sphingosine derivatives α-tocopherol and 0.30 g of propyl benzoate were stirred to prepare a beige cream. At this time, the prepared composition contained 29.1% by weight of phospholipids or sphingosine derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com