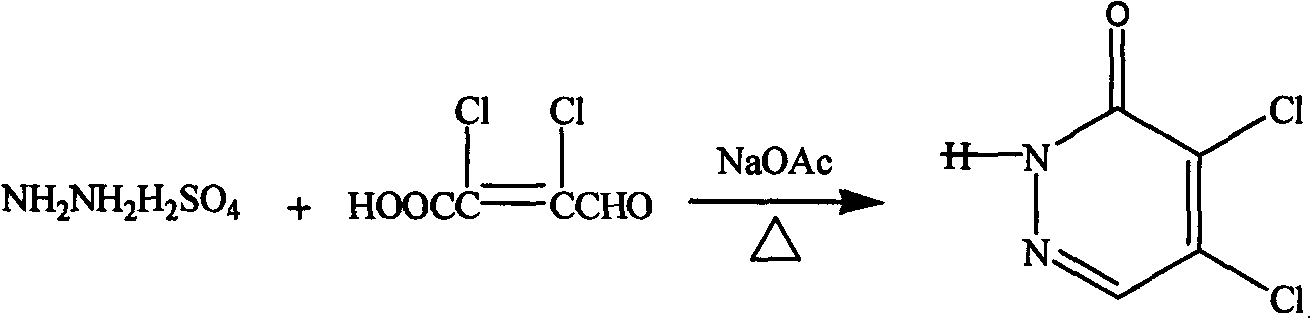Pyridazinone compound and synthesis method thereof
A technology of pyridazinone and compounds, which is applied in the field of inhibitors and their synthesis, can solve problems such as application limitations, and achieve the effects of wide application range and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
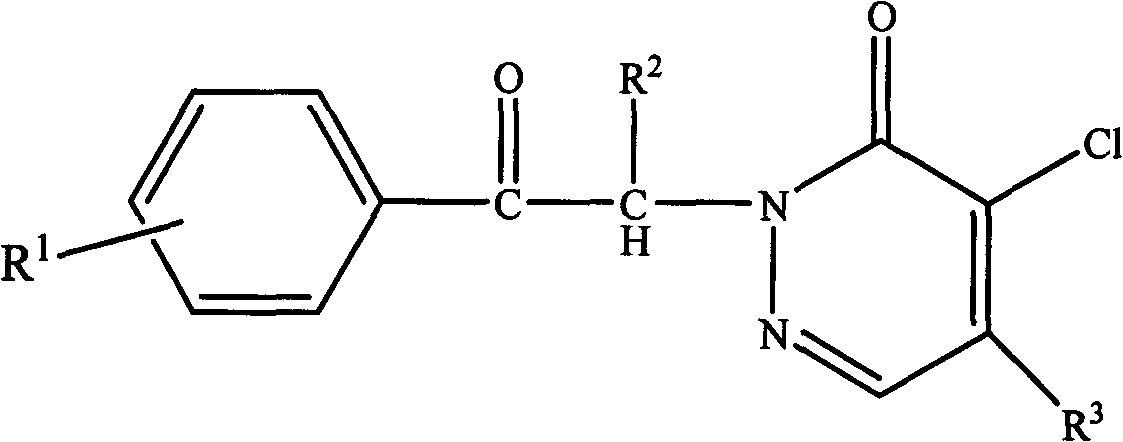
[0027] A pyridazinone compound, the compound molecular formula is as follows
[0028]
[0029] R 1 is methyl, R 2 for hydrogen, R 3 For morpholine, the synthetic method of this pyridazinone compound comprises the following steps:
[0030] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0031]
[0032] In a 250mL three-necked flask, add 50g of dichlorobutenalic acid and a small amount of water, stir to make an aqueous solution, then add 39g of hydrazine sulfate and 38.2g of sodium acetate, heat to 80-100°C, and react for 2 hours. After complete reaction, cool down, pour the reaction solution into cold water, a large amount of light yellow precipitate appears, filter it with suction, and dry it. The obtained product was recrystallized with absolute ethanol, and the yield was 89.5%.
[0033] (2) Preparation of 2-bromo-p-methylacetophenone
[0034]
[0035] In a 250mL three-necked flask with a condensation and drying device, add 70mL of toluene, 26.6g of anhydro...
Embodiment 2
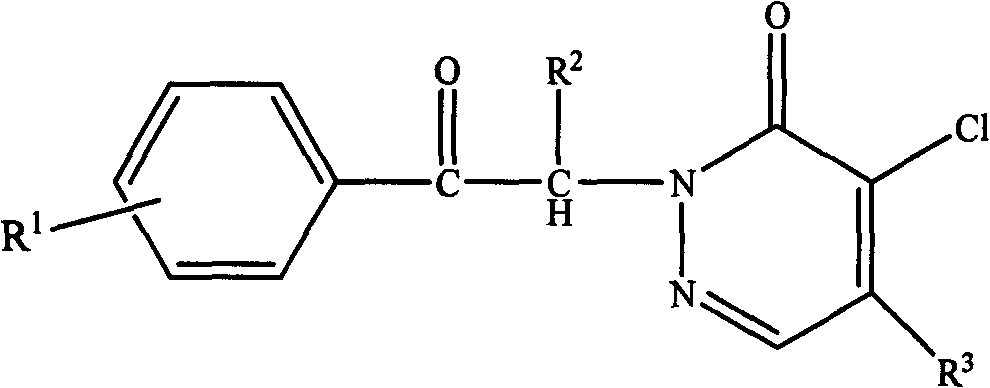
[0043] A pyridazinone compound, the compound molecular formula is as follows
[0044]
[0045] R 1 is chlorine, R 2 is ethyl, R 3 Be piperidine, the synthetic method of this pyridazinone compound comprises the following steps:
[0046] (1) Preparation of 2-bromo-1-p-chlorophenyl-1-butanone
[0047]
[0048] In a 250ml three-neck flask equipped with a condensation drying device, add 60ml of chlorobenzene and 26g of anhydrous AlCl3, stir, and slowly add 2-bromobutyryl bromide dropwise under ice-water cooling, and react at low temperature for 2 hours. Then rise to 35oC, heat preservation reaction for 3 hours. The reaction solution was poured into 200ml of ice water and stirred continuously. Separate the organic phase, wash the organic phase with water 6 to 7 times, and dry it with anhydrous MgSO4. Remove chlorobenzene, distill under reduced pressure, collect fractions at 162-164°C, yield 78.2%
[0049] (2) Preparation of 4,5-dichloro-2-[2-(4-chloro-phenyl)-2-oxo-ethy...
Embodiment 3
[0056] A preparation method of pyridazinone compound, comprising the following steps:
[0057] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0058] Mix di-chlorocrotoninic acid and hydrazine sulfate at a molar ratio of 1:1, and under the catalysis of sodium acetate, conduct condensation reaction at 80°C for 1 hour to obtain 4,5-dichloro-3(2H ) pyridazinone, wherein the molar ratio of di-chlorocrotonic acid to sodium acetate is 1:1;
[0059] (2) Preparation of α-halogenated-aromatic aliphatic mixed ketones
[0060] The substituted aromatic hydrocarbon ethylbenzene and α-bromoacetyl bromide are mixed in a molar ratio of 1:1, in AlCl 3 Under the catalysis of α-halogenated-aromatic aliphatic mixed ketones, the AlCl 3 The addition amount is that the molar ratio with ethylbenzene is 1: 1;
[0061] (3) Preparation of 2-substituted-3(2H)-pyridazinone
[0062] The 4,5-dichloro-3 (2H) pyridazinone prepared in step (1) and the α-halogenated-aromatic fatty mixed ketone prepared...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap