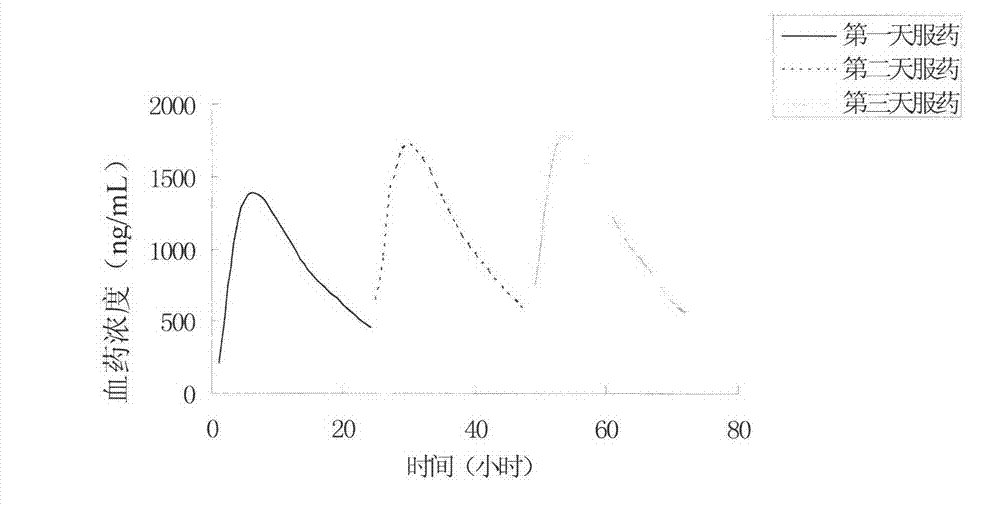
Gliclazide tablet (II) and preparation method thereof
A technology for gliclazide tablets and tablet compression, which is applied in the field of gliclazide tablets and its preparation, and can solve the problems of slow release speed of gliclazide sustained-release tablets, inability to stop taking medicine immediately, delayed drug effect, etc. , to achieve the effect of preventing hypoglycemia side effects, significant effect, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] components mg / tablet Grecht 40 Hydroxypropyl Methyl Cellulose (K15M) 10 Sodium carboxymethyl starch 5 starch 42 PVP K30 2 Magnesium stearate 1 total weight 100
[0031] Preparation method: sieve gliclazide, hydroxypropyl methylcellulose, sodium carboxymethyl starch, and starch respectively, dry mix for 10 minutes, add the binder povidone ethanol solution to make a soft material, granulate, and dry , granulated, added with magnesium stearate, mixed evenly, compressed into tablets, ready to be obtained.
[0032] The Gliclazide Tablets (II) prepared in this example were tested for drug release according to the method of Gliclazide Tablets (II) recorded in Part II of the Chinese Pharmacopoeia 2010 Edition, and the results were recorded in the following table:
[0033] time (hours) Cumulative percent of drug released 1 41.4% 2 64.7% 3 79.3% 4 87.8% 5 ...
Embodiment 2
[0035] components mg / tablet Grecht 40 Hydroxypropyl Methyl Cellulose (K15M) 5 Sodium carboxymethyl starch 4 pregelatinized starch 40 microcrystalline cellulose 10 Magnesium stearate 1 total weight 100
[0036] Preparation method: sieve gliclazide, hydroxypropyl methylcellulose, sodium carboxymethyl starch, pregelatinized starch and microcrystalline cellulose respectively, dry mix for 20 minutes, add magnesium stearate and mix evenly, directly Tablets, ready to use.
[0037]The gliclazide tablet (II) prepared by the present embodiment is carried out drug release assay as described in Example 1:
[0038] time (hours) Cumulative percent of drug released 1 38.5% 2 67.2% 3 82.1% 4 89.4% 5 95.3% 6 99.5%
Embodiment 3
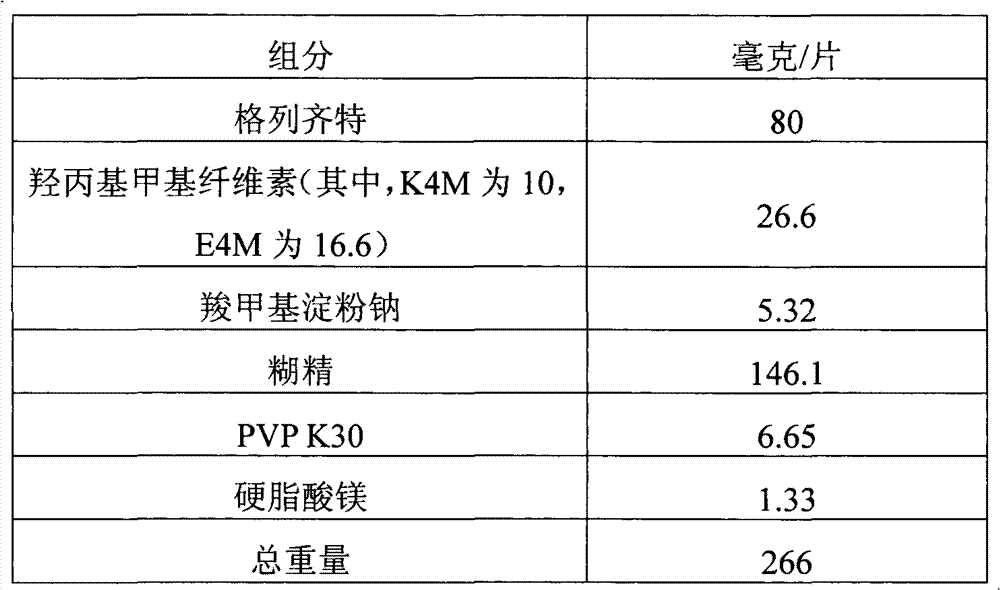
[0040]
[0041] Preparation method: sieve gliclazide, hydroxypropyl methylcellulose, sodium carboxymethyl starch and dextrin respectively, dry mix for 10 minutes, add binder povidone ethanol solution to make soft material, granulate, Dried, granulated, added magnesium stearate, mixed evenly, compressed into tablets, and obtained.
[0042] The gliclazide tablet (II) prepared by the present embodiment is carried out drug release assay as described in Example 1:
[0043] time (hours) Cumulative percent of drug released 1 40.8% 2 68.1% 3 83.0% 4 89.2% 5 94.9% 6 100.4%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com