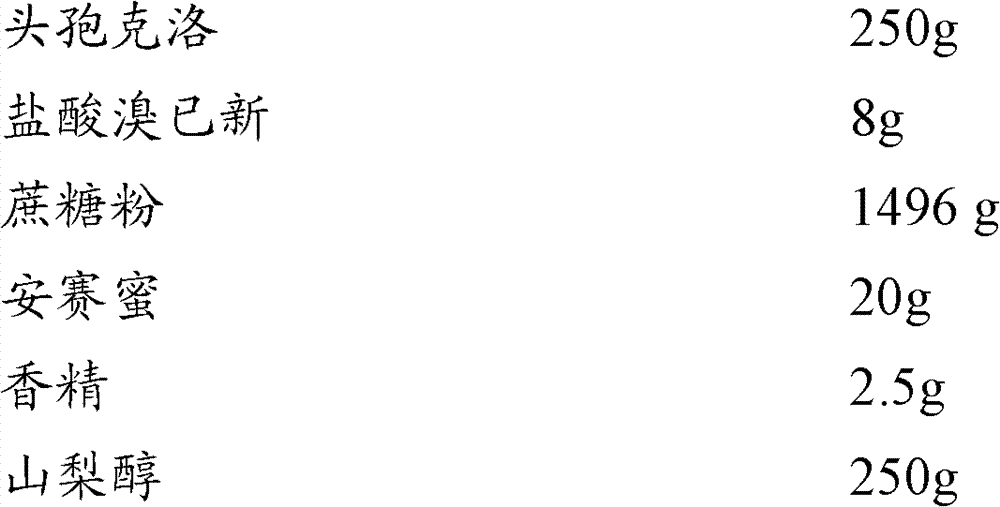Cefaclor composition particles and preparation method thereof
A technology of cefaclor and a composition, applied in the field of medicine, can solve problems such as poor dissolution rate, poor patient compliance, unfavorable swallowing, etc., and achieve the effects of enhancing antibacterial effect, improving drug dissolution rate, and avoiding drug agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Cefaclor composition granule of the present invention
[0018]
[0019] Preparation method: Take 250g of cefaclor, 8g of bromhexine hydrochloride, 1490g of sucrose and 20g of acesulfame potassium and mix in a high-speed granulator for 10 minutes, then add 250g of sorbitol, mix for 3 minutes, release the granules, and granulate with a shaking granulator , the prepared wet granules are transferred to a boiling dryer and dried at 65°C for 25 to 30 minutes; the prepared dry granules and essence 2.5g are mixed, sized and sieved to obtain the product.
Embodiment 2
[0020] Embodiment 2: the antibacterial action of cefaclor composition granule of the present invention
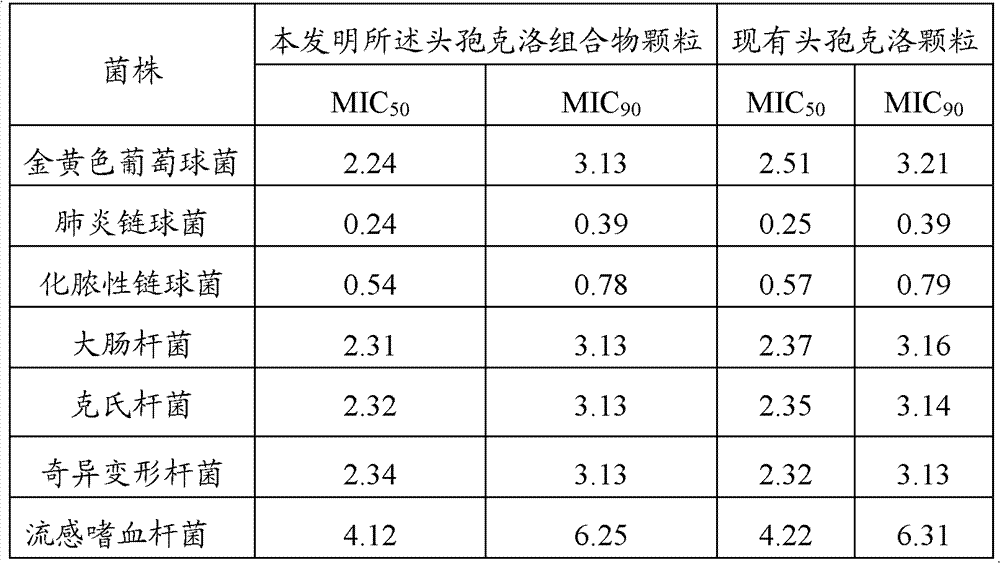
[0021] Adopt agar dilution method to measure the minimum inhibitory concentration (MIC) of cefaclor composition of the present invention and existing cefaclor granule, the two adopt different concentration dilution ranges to different bacterial strains, inoculate with multi-point seeder, The number of bacteria contained in each point is about 104CFU. Incubate at 37°C for 24 hours, and the results are shown in Table 1.
[0022] Table 1 Antibacterial test results
[0023]
[0024] As can be seen from the results in Table 1, the cefaclor composition granules of the present invention have stronger antibacterial activity, inhibiting the minimum inhibitory concentration (MIC, μg / mL) of about 90% bacterial strains: Staphylococcus aureus 3.13, Streptococcus pneumoniae 0.39 , Streptococcus pyogenes 0.78, Escherichia coli, Klebsiella, Proteus mirabilis 3.13, Haemophilus influenz...
Embodiment 3
[0025] Example 3: Clinical research on the antibacterial effect of the cefaclor composition granules of the present invention
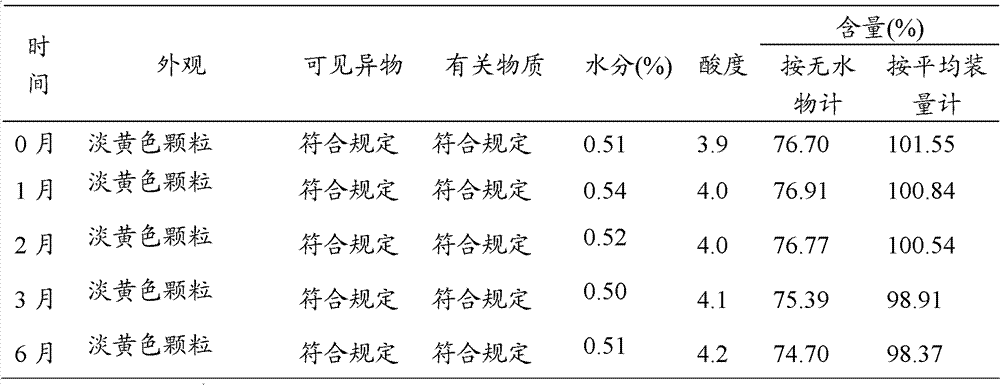
[0026] 2000 patients with respiratory tract infection, urinary tract infection and skin and soft tissue infection caused by sensitive bacteria were randomly divided into treatment group A, treatment group B and control group. Treatment group A gave existing compound cefaclor tablets on the basis of routine treatment, and treatment group B gave the cefaclor composition granules prepared in Example 1 of the present invention on the basis of routine treatment, 2 times a day, every day 300mg each time, the course of treatment was 10d, and the control group was given cephalexin. The bacterial clearance rate of each group was counted, and the results are shown in Table 2.
[0027] Table 2 Antibacterial effect clinical research
[0028]
control group
Treatment group A
Treatment group B
Bacterial clearance
/
93.6%
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com