Preparation method for 2,3,4-trimethoxy benzonitrile
A technology of trimethoxybenzonitrile and trimethoxybenzene, which is applied in the field of preparation of 2,3,4-trimethoxybenzonitrile, can solve the problems of high comprehensive cost and many reaction steps, and reduce production cost and steps Less, less expensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
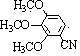
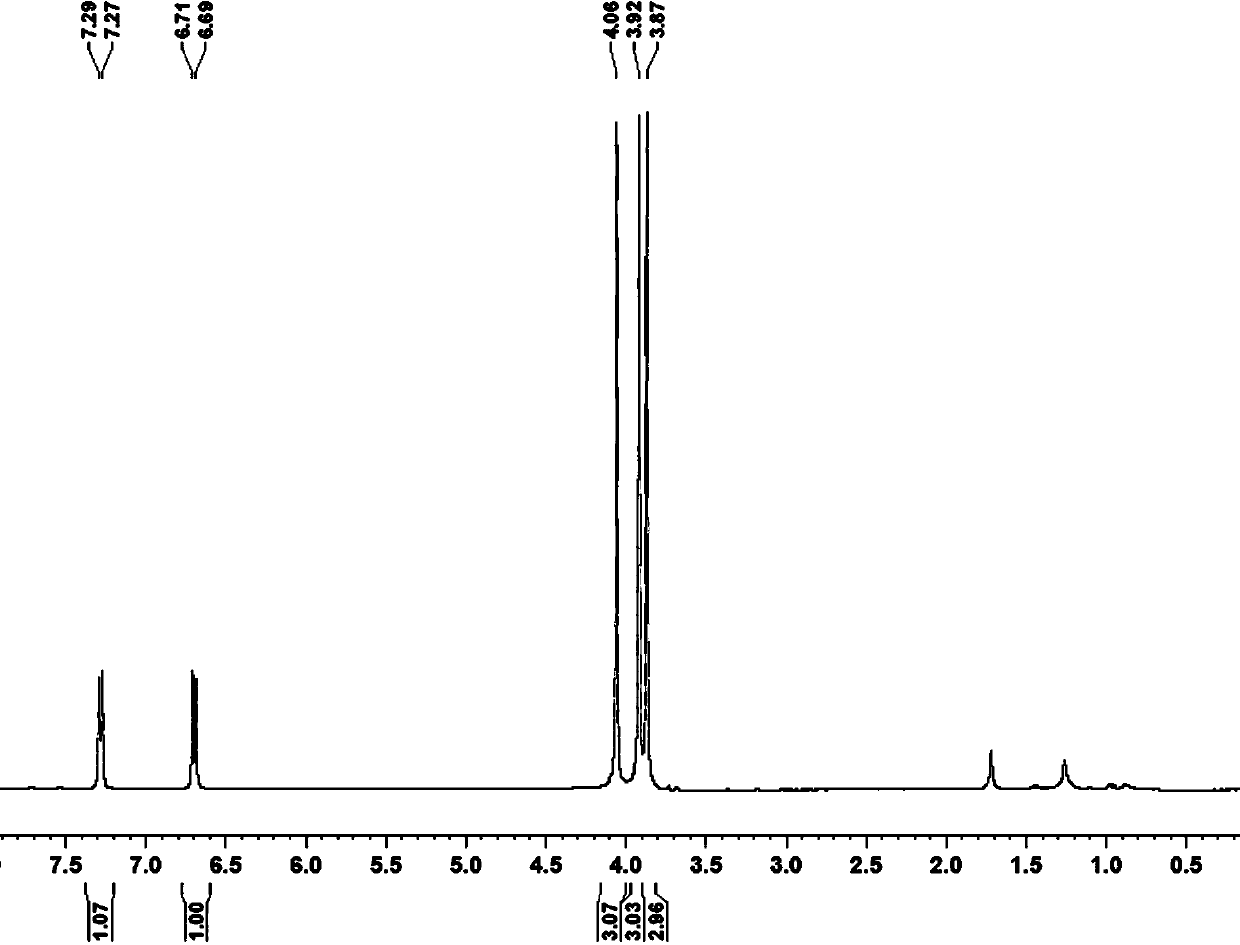
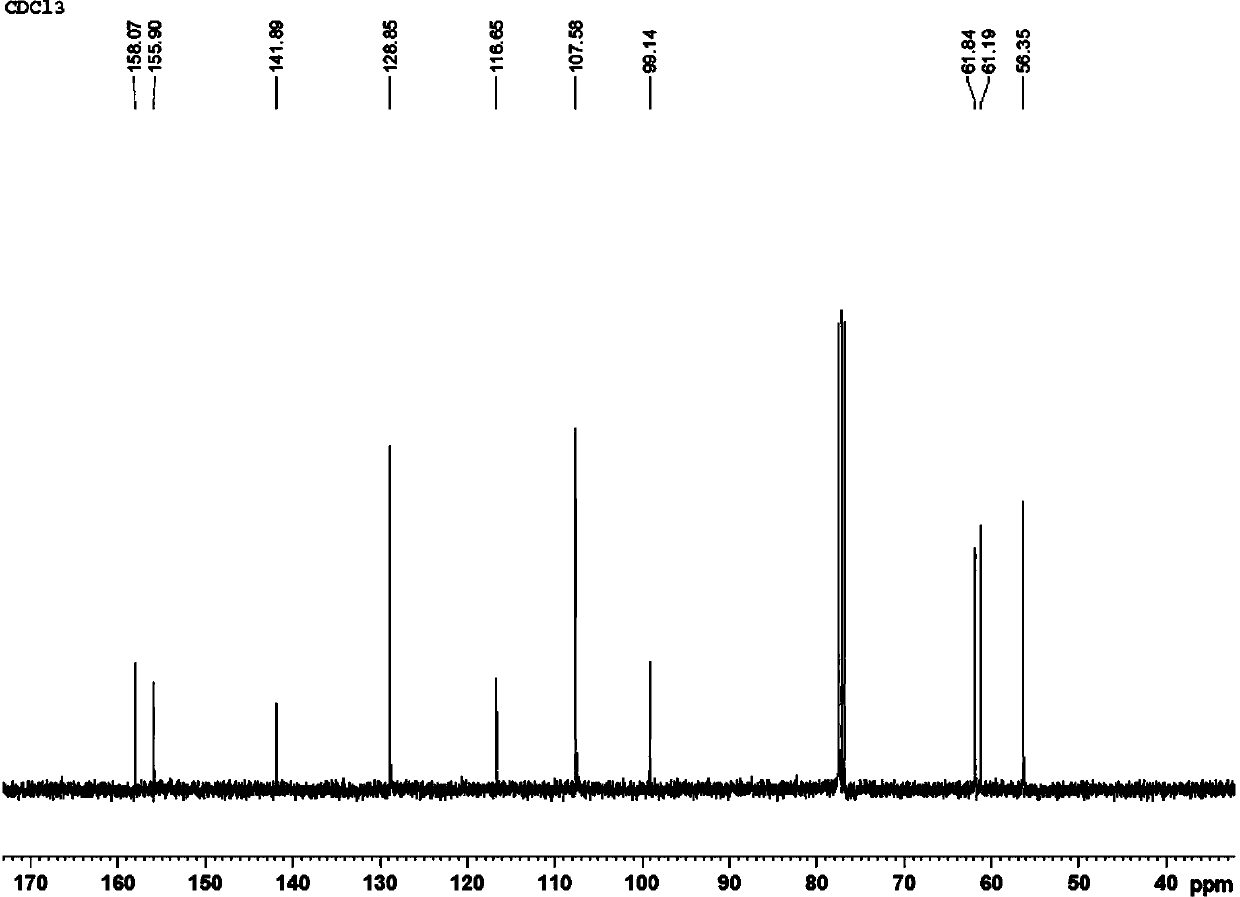
[0028] Add 0.5mmol 1,2,3-trimethoxybenzene, 0.25mmol I 2 , 0.4mmol copper chloride, 0.4mmol silver nitrate, 0.25mmol potassium ferricyanide, 1.5mL 1-n-butyl-3-methylimidazolium tetrafluoroborate. After the reaction tube was sealed, the reaction tube was placed in an oil bath preheated to 180° C., and magnetically stirred at 180° C. for 35 hours. After the reaction was completed, the reaction system was cooled to room temperature. The reacted mixture was purified by column chromatography. The obtained 2,3,4-trimethoxybenzonitrile product was a white solid with a yield of 72%. use 1 H-NMR and 13 C-NMR confirmed the structure of the product, which 1 H-NMR diagram and 13 C-NMR charts are shown in figure 1 and 2 . 1 H NMR (400 MHz, CDCl 3 ): δ (ppm) = 7.28 (d, J = 8.0 Hz, 1H), 6.70 (d, J = 8.0 Hz, 1H), 4.06 (s, 3H), 3.92 (s, 3H), 3.87 (s, 3H). 13 C NMR (400 MHz, CDCl 3 ): δ (ppm) = 158.1, 155.9, 141.9, 128.9, 116.7, 107.6, 99.1, 61.8, 61.1, 56.4.
Embodiment 2
[0030] Add 2.5mmol 1,2,3-trimethoxybenzene, 1.25mmol I 2 , 2mmol copper chloride, 2mmol silver nitrate, 1.25mmol potassium ferricyanide, 10mL 1-n-butyl-3-methylimidazolium tetrafluorophosphate. After the reaction tube was sealed, the reaction tube was placed in an oil bath preheated to 190° C., and magnetically stirred at 190° C. for 20 hours. After the reaction was completed, the reaction system was cooled to room temperature. The reacted mixture was purified by column chromatography. The obtained 2,3,4-trimethoxybenzonitrile product was a white solid with a yield of 53%. use 1 H-NMR and 13 C-NMR confirms the structure of product, and its spectrogram data is identical with embodiment 1.
Embodiment 3
[0032] Add 1 mmol 1,2,3-trimethoxybenzene, 0.5 mmol I 2 , 0.8mmol copper chloride, 0.8mmol silver nitrate, 0.5mmol potassium ferricyanide, 3mL 1-n-hexyl-3-methylimidazolium tetrafluoroborate. After the reaction tube was sealed, the reaction tube was placed in an oil bath preheated to 185°C, and magnetically stirred at 185°C for 25 hours. After the reaction was completed, the reaction system was cooled to room temperature. The reacted mixture was purified by column chromatography. The obtained 2,3,4-trimethoxybenzonitrile product was a white solid with a yield of 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com