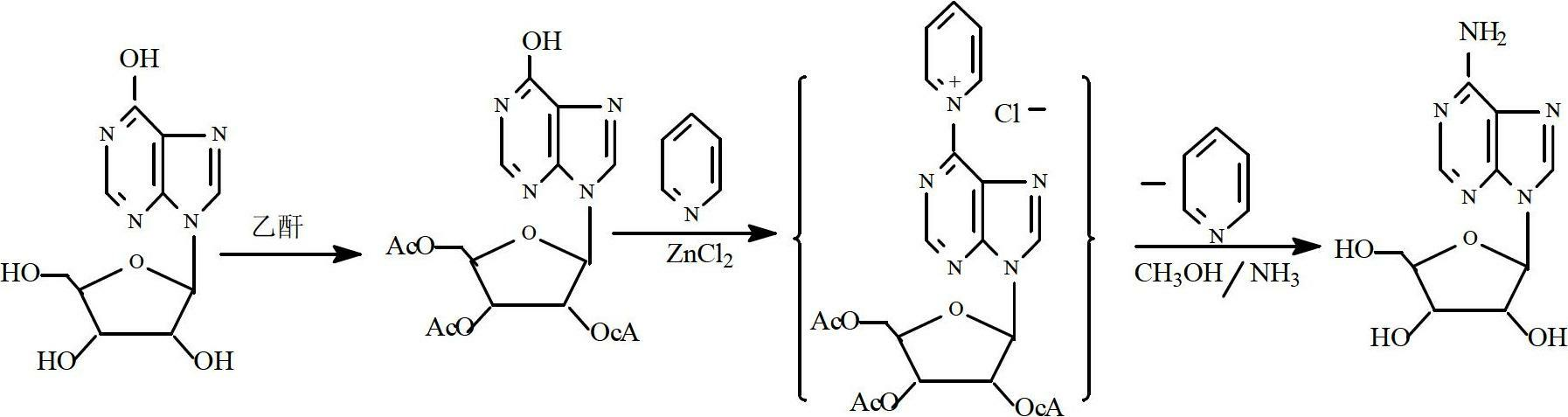
Technique for synthesizing adenosin by chemical method
A technology of chemical method and adenosine, which is applied in the technical field of adenosine synthesis by chemical method, can solve the problems of large pyridine usage, long process route, and low yield, so as to reduce equipment investment and material loss, shorten process flow, and improve The effect on product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Preparation of triacetyl inosine:
[0021] Install a thermometer and a condenser on a 1000ml four-necked reaction bottle, put in 200g inosine, 300ml acetic anhydride, and 2g anhydrous sodium acetate, heat up to reflux, react for 1.5 hours, recover excess acetic anhydride and the acetic acid produced, and use 200ml of cold water was washed until neutral, and vacuum-dried to obtain 286g of white crystals of triacetyl inosine, with a yield of 97.27%.
[0022] (2) Preparation of adenosine:
[0023] In a 1000ml autoclave, accurately add 50g of triacetyl inosine obtained in (1), add 500ml of anhydrous methanol, 0.4g of AlCl 3 1. 2ml of anhydrous pyridine, stirred and cooled to 0±2°C, passed ammonia to saturation, sealed, heated to 65±2°C, and reacted for 5 hours. After the reaction was completed, excess methanol and ammonia were recovered to obtain a crude product, which was purified to obtain 25.23 g of white crystals of adenosine, HPLC 99.78%, and a yield of 72.44% (c...
Embodiment 2
[0025] In a 1000ml autoclave, accurately add 50g of triacetyl inosine obtained in Example 1 (1), add 500ml of anhydrous methanol, 3g of ZnCl 2 , 5ml of anhydrous pyrrole, stirred and cooled to 0±2°C, passed ammonia to saturation, sealed, heated to 78±2°C, and reacted for 5 hours. After the reaction was completed, excessive methanol and ammonia were recovered to obtain the crude product, which was purified to obtain adenocarcinamide Glycoside white crystals 25.56g, HPLC 99.62%, yield 73.38% (calculated as inosine).
Embodiment 3
[0027] In a 1000ml autoclave, accurately add 50g of triacetyl inosine obtained in Example 1 (1), add 500ml of anhydrous methanol, 2g of anhydrous SnCl 4 , 3ml of anhydrous pyridine, stirring to cool down to 0±2°C, pass ammonia to saturation, seal, heat up to 70±2°C, react for 4 hours, after the reaction is complete, recover excess methanol and ammonia to obtain the crude product, which is refined to obtain adenocarcinamide Glycoside white crystals 25.78g, HPLC 99.62%, yield 74.01% (calculated as inosine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com