Glipizide sustained release tablets
A technology for glipizide and sustained-release tablets, which is applied in the field of glipizide sustained-release tablets, can solve the problems of high viscosity of the mixture, difficulty in reaching the standard of release, high particle hardness, etc., and achieves good compressibility and tightness of particles. Appropriate, smooth granulation results
Inactive Publication Date: 2012-12-19
DISHA PHARMA GRP +1
View PDF0 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
During the preparation process of Glipizide Sustained-release Tablets, if the formulation design is improper, there will be problems that the release rate is difficult to reach the standard. At the same time, there will be problems such as high viscosity of the mixture and high particle hardness during the granulation process.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
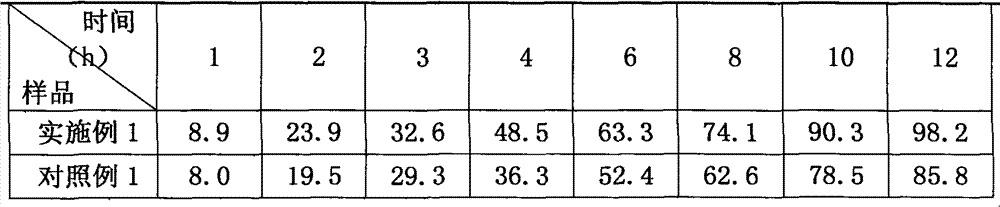
Embodiment 1
[0015] Embodiment 1, prescription: glipizide 5g, hypromellose (K15M) 26g, microcrystalline cellulose 30g, lactose 60g, sodium carboxymethylcellulose 6.5g, hypromellose (E5) amount, Magnesium stearate 0.60g, prepare 1000 tablets by the preparation method described in the technical scheme part.
[0016] The granulation of this prescription is smooth, the granules are suitable for tightness, and the compressibility is good.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to glipizide sustained release tablets for treating diabetes mellitus. The technical scheme provided by the invention is as follows: the glipizide sustained release tablets are characterized in that every 1,000 glipizide sustained release tablets contain 5g of glipizide, 26g of hydroxypropyl methylcellulose (K15M) and 6.5g of sodium carboxymethylcellulose.
Description
[0001] Technical Field The present invention relates to a glipizide sustained-release tablet for treating diabetes. Background technique [0002] Diabetes is an epidemic in the 21st century, and it is the third largest disease after cardiovascular disease and cancer. In my country, with the improvement of people's material life and medical diagnosis level, the number of diabetic patients has increased year by year, and it has reached about 30 million at present. About 95% of them belong to type 2 diabetes. Sulfonylureas (SU) are oral hypoglycemic drugs with the earliest application, the largest variety, and the most extensive clinical application. Among them, glipizide has the fastest development speed. Since the second half of 2003, the product’s The sales surpassed the once famous gliclazide, becoming the sulfonylurea hypoglycemic drug with the highest sales. Glipizide preparations that have been marketed include regular tablets and sustained-release tablets. [0003] Gli...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/22A61K31/64A61K47/38A61P3/10
Inventor 丁艳龙连清王寅峰
Owner DISHA PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com