Hindered amine compound and preparation method thereof
A technology for hindered amines and compounds, applied in the field of hindered amine compounds and their preparation, can solve the problems of accelerated photooxidative degradation of materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
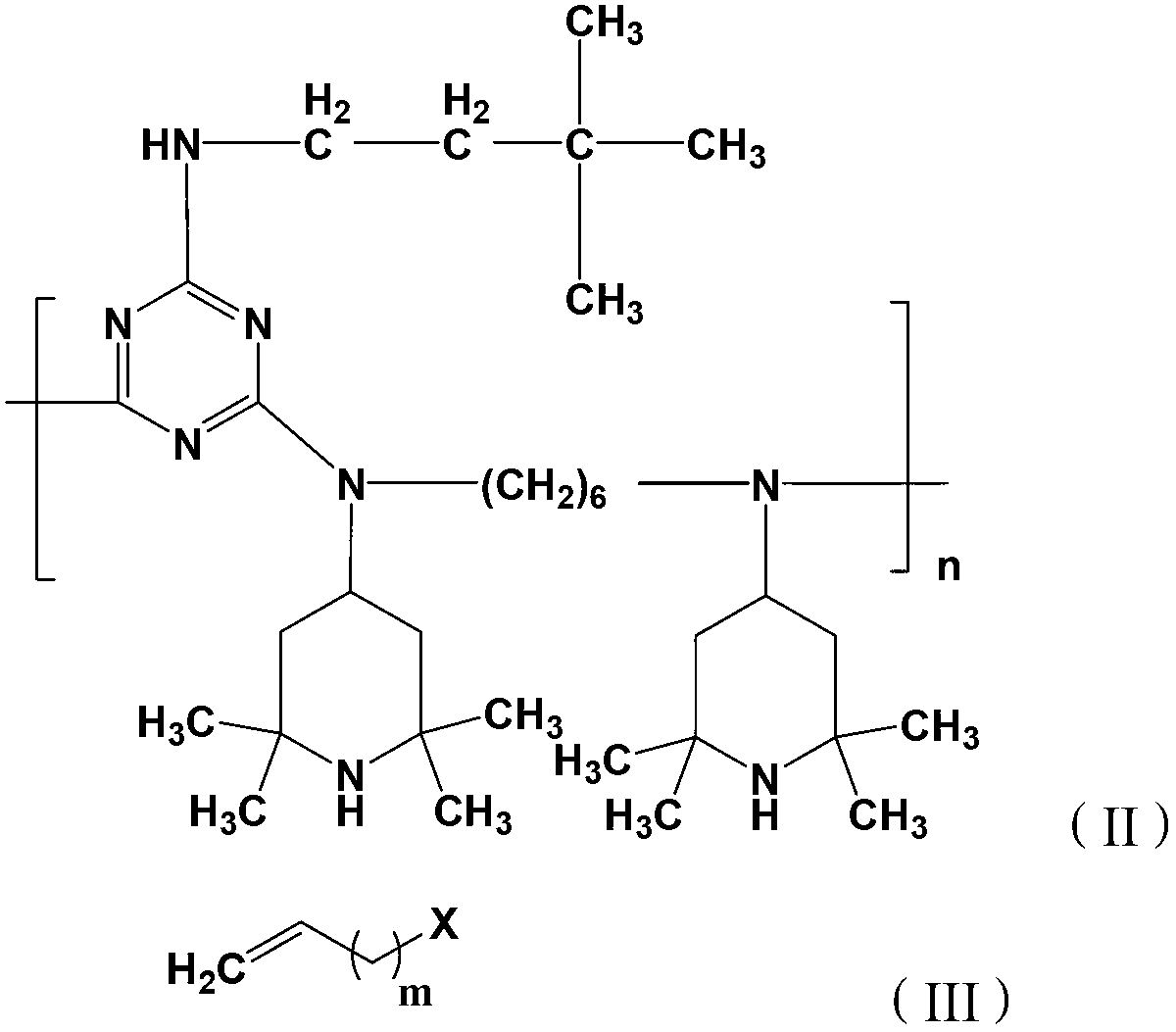
[0027] The present invention also provides a preparation method for the aforementioned hindered amine compound, comprising the following steps: A) reacting the compound of formula (II) with a protective reagent in a protective atmosphere to obtain an amino-protected intermediate product; B) adding the amino The protected intermediate product is reacted with the terminal alkene compound of the formula (III), and the hindered amine compound of the formula (I) is obtained after deprotection. The protective atmosphere is a protective atmosphere well known to those skilled in the art, preferably nitrogen.
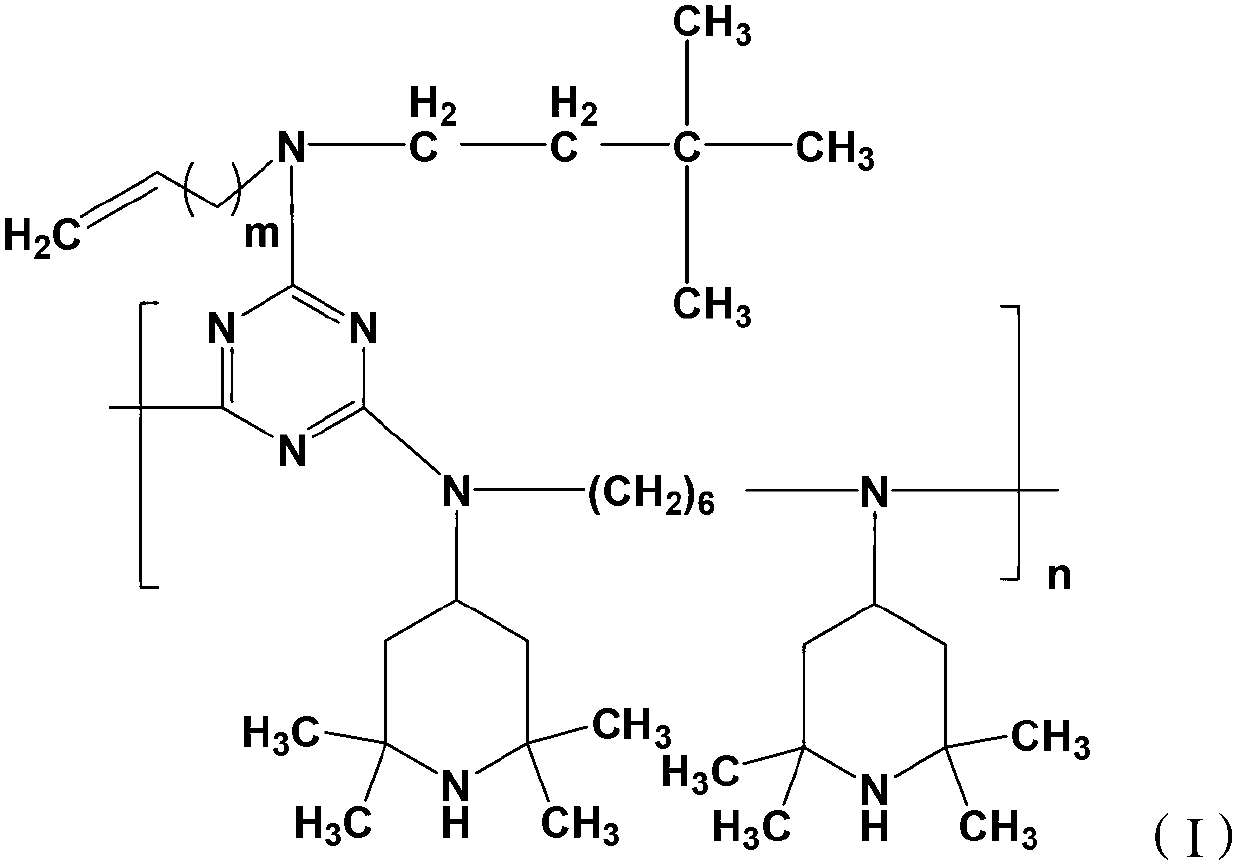
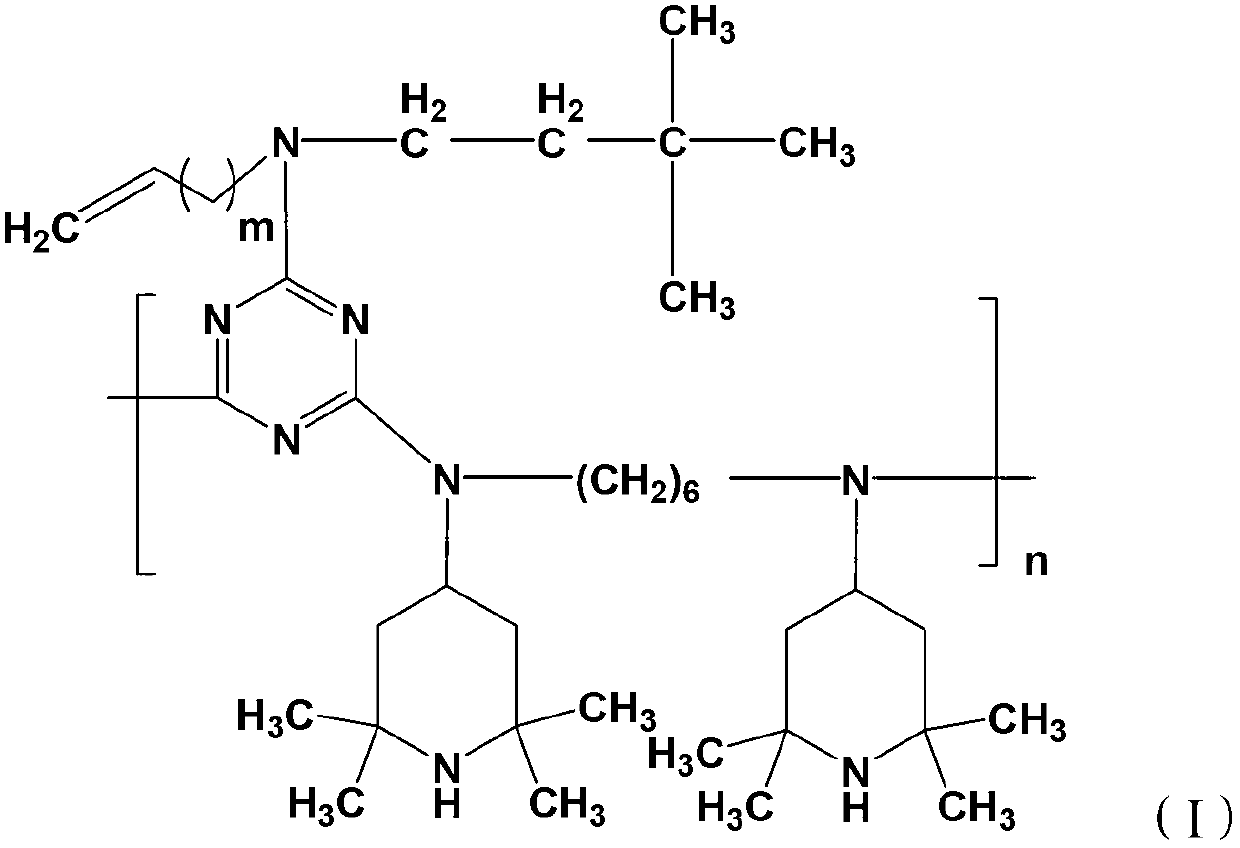
[0028]
[0029] Wherein, m=1~9, n=3~10, X is Cl, Br or I. m is preferably 1-6, more preferably 2-4.
[0030] The compound of the formula (II) is a light stabilizer known to those skilled in the art as GW-944.
[0031] In step A, the molar ratio of the compound of the formula (II) to the protecting reagent is 1:2~2.5, preferably 1:2~2.2. The reaction time of the reaction is...
Embodiment 1
[0049] The preparation of embodiment 1 GW-944
[0050] 1.1 Cool down 120ml of toluene in an ice bath to below 10°C, add 40g of cyanuric chloride, stir, slowly add 50ml of toluene solution dissolved in 27.52g of tert-octylamine dropwise, after 2 hours of reaction, dropwise add 20% cyanuric chloride 50ml of NaOH aqueous solution was reacted for 2h, allowed to stand, and the organic phase was separated and washed with 10% NaOH aqueous solution to obtain an organic phase containing intermediate product IV.
[0051] 1.2 Add the organic phase containing 0.2 mol of intermediate product IV obtained in 1.1, 0.2 mol of hexamethylenediamine piperidine and 50 ml of NaOH aqueous solution with a mass concentration of 20% into a 500 ml autoclave, replace the air in the autoclave with nitrogen three times, and fill with nitrogen To a pressure of 2MPa, heat to 60°C, stir for 4 hours, heat up to 180°C, react for 6 hours, cool down, filter, wash the organic phase with 10% NaOH aqueous solution, ...
Embodiment 2
[0057] Under the condition of nitrogen protection, mix and stir 0.2 mol of GW-944 obtained in 1.2, 0.4 mol of di-tert-butyl dicarbonate, 0.18 mol of potassium carbonate and 120 ml of dichloromethane. After 4 hours of reaction, add 0.3 mol of allyl bromide , Continue to react for 10h. Add dilute hydrochloric acid to adjust the pH value of the reaction solution to 5.0, remove the solvent under reduced pressure, extract with ether, anhydrous Na 2 SO 4 After drying and purification by n-hexane / ethyl acetate column chromatography, the hindered amine compound with the structure of formula (I) was obtained, and its average relative molecular mass was 2960.
[0058] The hindered amine compounds of the formula (I) structure obtained in Example 2 were analyzed by nuclear magnetic resonance to obtain their hydrogen spectrum and carbon spectrum. The results are as follows:
[0059] 1 HNMR (CDCl 3 ,ppm): 5.87(-C=CH), 5.19(-C=CH), 3.73(-CH 2 ), 3.49 (-CH 2 ),2.63(-CH),2.44(-CH),2.26(-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com