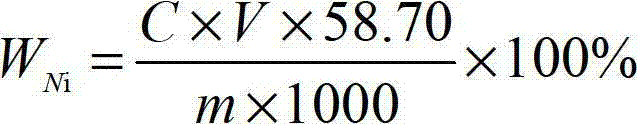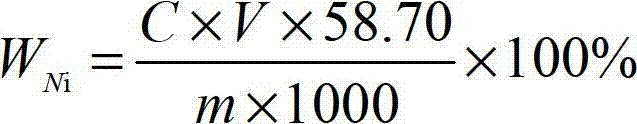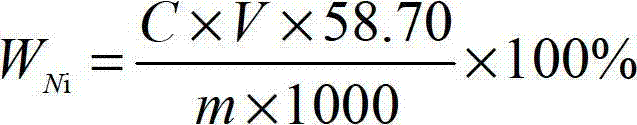Method for measuring content of nickel in ferronickel
A technology of nickel content and nickel-iron, which is applied in the preparation of test samples, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, can solve the problems of long cycle time, inability to detect, Not suitable for factory production testing and other issues, to achieve accurate measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045] First, weigh a certain amount of ferronickel sample (accurate to 0.00001g) for the test, and weigh the sample according to Table 1.
[0046] Table 1
[0047] Nickel content range (%)
Sample weight (g)
≥1.00~2.00
2.00
>2.00~7.00
1.00
>7.00~15.00
0.50
>15.00~25.00
0.30
>25.00~38.00
0.20
>38.00~85.00
0.10
[0048] Place the weighed ferronickel sample for the test in a 600mL beaker, add 20mL of nitric acid with a concentration of 64% and hydrochloric acid with a concentration of 35% according to the volume ratio of 1:1 mixed nitrate mixed acid, heat at low temperature until the sample is completely Dissolve, add 10mL of perchloric acid with a concentration of 68%, heat until fumes of perchloric acid appear, remove the beaker from the heating device, add 100mL of water, keep warm to completely dissolve the salt in the mixture, then cool to room temperature, 2 g of citric ...
example 2
[0065] First, weigh a certain amount of ferronickel sample (accurate to 0.00001g) for the test, and weigh the sample according to Table 1.
[0066] Place the weighed ferronickel sample for the test in a 600mL beaker, add 20mL of nitric acid with a concentration of 60% and hydrochloric acid with a concentration of 30% according to the volume ratio of 1:2 mixed nitrate mixed acid, heat at low temperature until the sample is completely Dissolve, add 10mL of perchloric acid with a concentration of 65%, heat until fumes of perchloric acid appear, remove the beaker from the heating device, add 90mL of water, keep warm to completely dissolve the salt in the mixture, then cool to room temperature, 2 g of citric acid was added to the mixture, and then 20% ammonia water was added to neutralize the mixture until the pH value of the mixture was 8.5 with an excess of 18 mL, and diluted with water to 300 mL. If the ferronickel sample contains chromium, the chromium needs to be completely ox...
example 3
[0083] First, weigh a certain amount of ferronickel sample (accurate to 0.00001g) for the test, and weigh the sample according to Table 1.
[0084] Place the weighed ferronickel sample for the test in a 600mL beaker, add 20mL of nitric acid with a concentration of 68% and hydrochloric acid with a concentration of 38% according to the volume ratio of 1:4, and heat at low temperature until the sample is completely Dissolve, add 10mL of perchloric acid with a concentration of 72%, heat until fumes of perchloric acid appear, remove the beaker from the heating device, add 110mL of water, keep warm to completely dissolve the salt in the mixture, then cool to room temperature, 2 g of citric acid was added to the mixture, and then 28% ammonia water was added to neutralize the mixture until the pH value of the mixture was 9.5 with an excess of 12 mL, and diluted with water to 300 mL. If the ferronickel sample contains chromium, the chromium needs to be completely oxidized.
[0085] If...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com