hnRNP A2* protein, nucleic acid for coding protein and application thereof
A protein and nucleic acid technology, applied in the field of hnRNP A2* protein, can solve the problems of enhancing G-quadruplex substrate extension, etc., to achieve the effects of enhancing catalytic activity and progress, extending telomeres, and solving cell expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] The principles and features of the present invention are described below, and the examples given are only for explaining the present invention, and are not intended to limit the scope of the present invention.
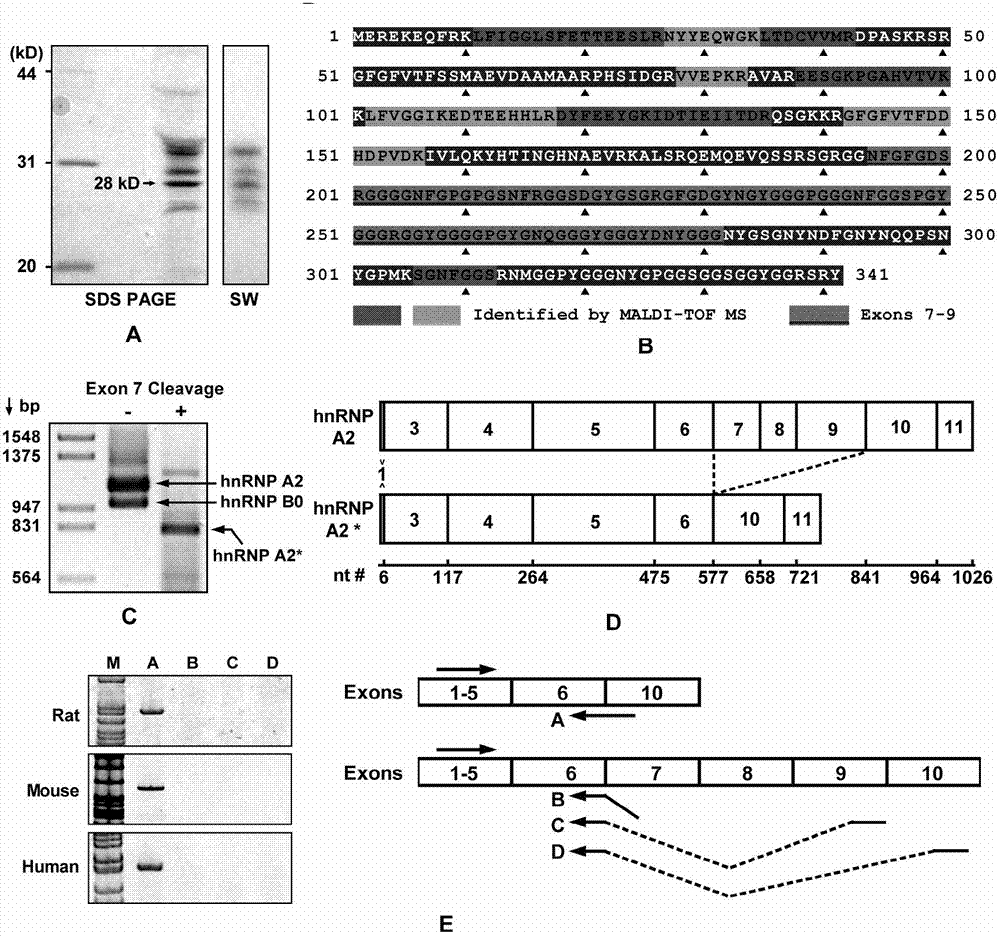
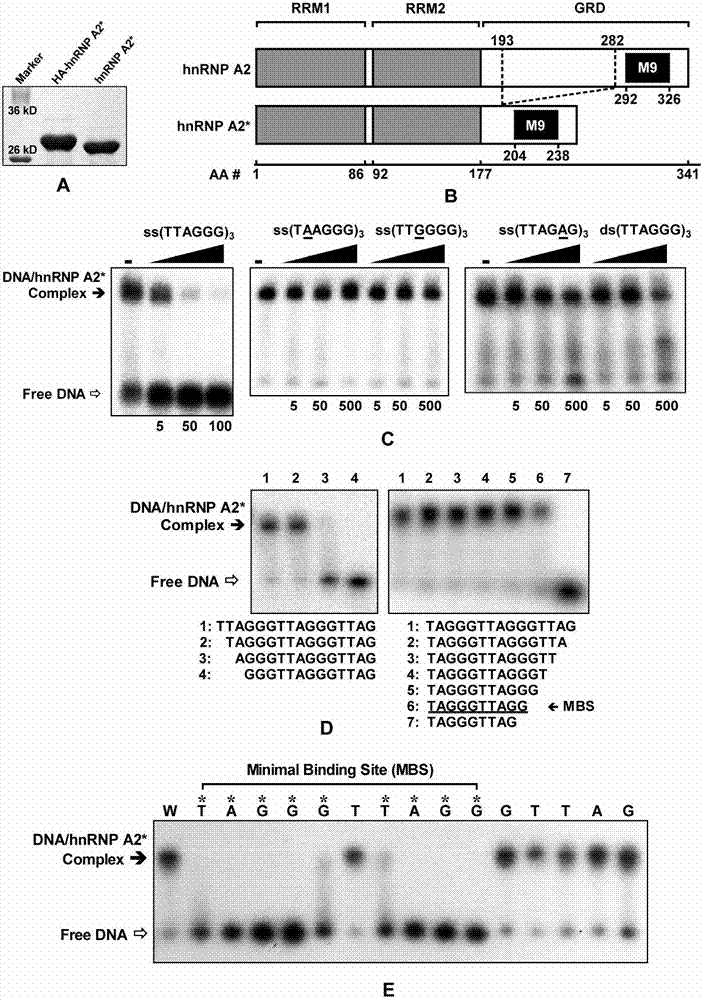
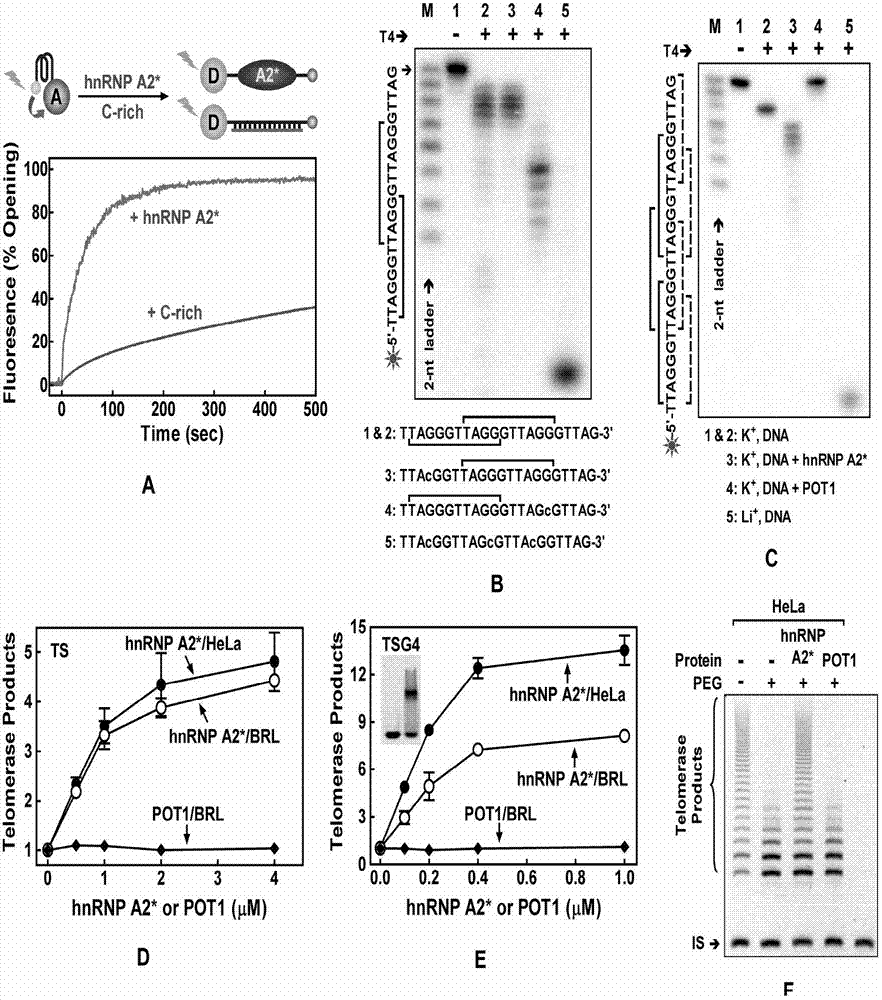
[0036] The present invention illustrates the research idea of the present invention through the following experiments, reveals the separation and purification process of hnRNP A2* protein, analyzes the principle of hnRNP A2* to untie the DNA quadruplex structure, and so on.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com